A New Developmental Mechanism for the Separation of the Mammalian
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Mammary Gland and Its Origin During Synapsid Evolution
P1: GMX Journal of Mammary Gland Biology and Neoplasia (JMGBN) pp749-jmgbn-460568 January 9, 2003 17:51 Style file version Nov. 07, 2000 Journal of Mammary Gland Biology and Neoplasia, Vol. 7, No. 3, July 2002 (C 2002) The Mammary Gland and Its Origin During Synapsid Evolution Olav T. Oftedal1 Lactation appears to be an ancient reproductive trait that predates the origin of mammals. The synapsid branch of the amniote tree that separated from other taxa in the Pennsylva- nian (>310 million years ago) evolved a glandular rather than scaled integument. Repeated radiations of synapsids produced a gradual accrual of mammalian features. The mammary gland apparently derives from an ancestral apocrine-like gland that was associated with hair follicles. This association is retained by monotreme mammary glands and is evident as ves- tigial mammary hair during early ontogenetic development of marsupials. The dense cluster of mammo-pilo-sebaceous units that open onto a nipple-less mammary patch in monotremes may reflect a structure that evolved to provide moisture and other constituents to permeable eggs. Mammary patch secretions were coopted to provide nutrients to hatchlings, but some constituents including lactose may have been secreted by ancestral apocrine-like glands in early synapsids. Advanced Triassic therapsids, such as cynodonts, almost certainly secreted complex, nutrient-rich milk, allowing a progressive decline in egg size and an increasingly altricial state of the young at hatching. This is indicated by the very small body size, presence of epipubic bones, and limited tooth replacement in advanced cynodonts and early mammali- aforms. Nipples that arose from the mammary patch rendered mammary hairs obsolete, while placental structures have allowed lactation to be truncated in living eutherians. -

Miocene Mammal Reveals a Mesozoic Ghost Lineage on Insular New Zealand, Southwest Pacific
Miocene mammal reveals a Mesozoic ghost lineage on insular New Zealand, southwest Pacific Trevor H. Worthy*†, Alan J. D. Tennyson‡, Michael Archer§, Anne M. Musser¶, Suzanne J. Hand§, Craig Jonesʈ, Barry J. Douglas**, James A. McNamara††, and Robin M. D. Beck§ *School of Earth and Environmental Sciences, Darling Building DP 418, Adelaide University, North Terrace, Adelaide 5005, South Australia, Australia; ‡Museum of New Zealand Te Papa Tongarewa, P.O. Box 467, Wellington 6015, New Zealand; §School of Biological, Earth and Environmental Sciences, University of New South Wales, New South Wales 2052, Australia; ¶Australian Museum, 6-8 College Street, Sydney, New South Wales 2010, Australia; ʈInstitute of Geological and Nuclear Sciences, P.O. Box 30368, Lower Hutt 5040, New Zealand; **Douglas Geological Consultants, 14 Jubilee Street, Dunedin 9011, New Zealand; and ††South Australian Museum, Adelaide, South Australia 5000, Australia Edited by James P. Kennett, University of California, Santa Barbara, CA, and approved October 11, 2006 (sent for review July 8, 2006) New Zealand (NZ) has long been upheld as the archetypical Ma) dinosaur material (13) and isolated moa bones from marine example of a land where the biota evolved without nonvolant sediments up to 2.5 Ma (1, 14), the terrestrial record older than terrestrial mammals. Their absence before human arrival is mys- 1 Ma is extremely limited. Until now, there has been no direct terious, because NZ was still attached to East Antarctica in the Early evidence for the pre-Pleistocene presence in NZ of any of its Cretaceous when a variety of terrestrial mammals occupied the endemic vertebrate lineages, particularly any group of terrestrial adjacent Australian portion of Gondwana. -

Versión Disponible En PDF
nicolás r chimento, Federico L agnolin y Fernando e novas Museo Argentino de Ciencias Naturales Bernardino Rivadavia Necrolestes un mamífero patagónico que sobrevivió a la extinción de los dinosaurios Un descubrimiento patagónico desembocadura del río Santa Cruz, descubrieron esque- letos fósiles prácticamente completos del Necrolestes. El es- En 1891, Florentino Ameghino (1854-1911) dio a tudio de esos esqueletos llevó a pensar que se trataba de conocer unos restos fósiles encontrados por su hermano un mamífero muy arcaico en la historia de la evolución, Carlos (1865-1936) en las barrancas de Monte Obser- más que un ancestro de los topos, como había supuesto vación, en la provincia de Santa Cruz, en yacimientos Ameghino. Por determinados rasgos se pensó que podía de unos 17 millones de años de antigüedad. Determinó haber sido un marsupial, es decir, un pariente lejano de las que pertenecían a un pequeño –escasos 10cm de largo, comadrejas, los canguros y los coalas actuales. del hocico a la cola– y desconocido mamífero extin- Ciertos investigadores aceptaron esta última hipótesis guido. Estudió los diminutos huesos y consideró que de parentesco, pero otros se mostraron escépticos acerca el animal habría sido un pariente lejano de los topos de ella y se inclinaron por considerar inciertas las rela- africanos vivientes. Le dio el nombre científicoN ecrolestes ciones genealógicas del diminuto mamífero. Así, su po- patagonensis, es decir, ladrón de tumbas de la Patagonia, en sición en el árbol evolutivo de los mamíferos fue objeto alusión a sus hábitos excavadores. El hallazgo, publica- de debate durante gran parte del siglo XX. Para algunos, do por Ameghino en el número de la Revista Argentina de era pariente lejano de las mulitas; otros seguían pensan- Historia Natural citado entre las lecturas sugeridas, atrajo do que podía estar relacionado con los topos, y para un la atención del ámbito científico, ya que hasta ese mo- tercer grupo, sus vínculos eran con los marsupiales aus- mento en Sudamérica no se habían encontrado restos tralianos. -

Recent Advances in Studies on Mesozoic and Paleogene Mammals in China
Vol.24 No.2 2010 Paleomammalogy Recent Advances in Studies on Mesozoic and Paleogene Mammals in China WANG Yuanqing* and NI Xijun Institute of Vertebrate Paleontology and Paleoanthropology, CAS, Beijing 10004, China ike in other fields of paleontology, research in from the articular of the lower jaw and the quadrate of the paleomammalogy mainly falls into two aspects. cranium following the process of reduction and detachment LOne is related to the biological nature of fossil from the reptilian mandible, are new elements of the bony mammals, such as their systematics, origin, evolution, chain in the mammalian middle ear. The appearance of phylogenetic relationships, transformation of key features mammalian middle ear allows mammals to hear the sound and paleobiogeography, and the other is related to the of higher frequencies than reptiles do. Generally speaking, geological context, involving biostratigraphy, biochronology, a widely accepted hypothesis is that mammals originated faunal turnover and its relations to the global and regional from a certain extinct reptilian group. The formation and environmental changes. In the last decade, a number of development of the definitive mammalian middle ear well-preserved mammalian specimens were collected from (DMME) has thus become one of the key issues in the study different localities around the country. Such discoveries have of mammalian evolution and has drawn the attention from provided significant information for understanding the early many researchers for many years. evolution of mammals and reconstructing the phylogeny of Developmental biological studies have proven the early mammals. function of the Meckel’s cartilage and its relationship to Studies of Chinese Mesozoic mammals achieved the ear ossicles during the development of mammalian remarkable progress in the past several years. -

The Mesozoic Era Alvarez, W.(1997)
Alles Introductory Biology: Illustrated Lecture Presentations Instructor David L. Alles Western Washington University ----------------------- Part Three: The Integration of Biological Knowledge Vertebrate Evolution in the Late Paleozoic and Mesozoic Eras ----------------------- Vertebrate Evolution in the Late Paleozoic and Mesozoic • Amphibians to Reptiles Internal Fertilization, the Amniotic Egg, and a Water-Tight Skin • The Adaptive Radiation of Reptiles from Scales to Hair and Feathers • Therapsids to Mammals • Dinosaurs to Birds Ectothermy to Endothermy The Evolution of Reptiles The Phanerozoic Eon 444 365 251 Paleozoic Era 542 m.y.a. 488 416 360 299 Camb. Ordov. Sil. Devo. Carbon. Perm. Cambrian Pikaia Fish Fish First First Explosion w/o jaws w/ jaws Amphibians Reptiles 210 65 Mesozoic Era 251 200 180 150 145 Triassic Jurassic Cretaceous First First First T. rex Dinosaurs Mammals Birds Cenozoic Era Last Ice Age 65 56 34 23 5 1.8 0.01 Paleo. Eocene Oligo. Miocene Plio. Ple. Present Early Primate First New First First Modern Cantius World Monkeys Apes Hominins Humans A modern Amphibian—the toad A modern day Reptile—a skink, note the finely outlined scales. A Comparison of Amphibian and Reptile Reproduction The oldest known reptile is Hylonomus lyelli dating to ~ 320 m.y.a.. The earliest or stem reptiles radiated into therapsids leading to mammals, and archosaurs leading to all the other reptile groups including the thecodontians, ancestors of the dinosaurs. Dimetrodon, a Mammal-like Reptile of the Early Permian Dicynodonts were a group of therapsids of the late Permian. Web Reference http://www.museums.org.za/sam/resource/palaeo/cluver/index.html Therapsids experienced an adaptive radiation during the Permian, but suffered heavy extinctions during the end Permian mass extinction. -

Mammal Evolution
Mammal Evolution Geology 331 Paleontology Triassic synapsid reptiles: Therapsids or mammal-like reptiles. Note the sprawling posture. Mammal with Upright Posture From Synapsids to Mammals, a well documented transition series Carl Buell Prothero, 2007 Synapsid Teeth, less specialized Mammal Teeth, more specialized Prothero, 2007 Yanoconodon, Lower Cretaceous of China Yanoconodon, Lower Cretaceous of China, retains ear bones attached to the inside lower jaw Morganucodon Yanoconodon = articular of = quadrate of Human Ear Bones, or lower reptile upper reptile Auditory Ossicles jaw jaw Cochlea Mammals have a bony secondary palate Primary Palate Reptiles have a soft Secondary Palate secondary palate Reduction of digit bones from Hand and Foot of Permian Synapsid 2-3-4-5-3 in synapsid Seymouria ancestors to 2-3-3-3-3 in mammals Human Hand and Foot Class Mammalia - Late Triassic to Recent Superorder Tricodonta - Late Triassic to Late Cretaceous Superorder Multituberculata - Late Jurassic to Early Oligocene Superorder Monotremata - Early Cretaceous to Recent Superorder Metatheria (Marsupials) - Late Cretaceous to Recent Superorder Eutheria (Placentals) - Late Cretaceous to Recent Evolution of Mammalian Superorders Multituberculates Metatheria Eutheria (Marsupials) (Placentals) Tricodonts Monotremes . Live Birth Extinct: . .. Mammary Glands? Mammals in the Age of Dinosaurs – a nocturnal life style Hadrocodium, a lower Jurassic mammal with a “large” brain (6 mm brain case in an 8 mm skull) Were larger brains adaptive for a greater sense of smell? Big Brains and Early Mammals July 14, 2011 The Academic Minute http://www.insidehighered.com/audio/academic_pulse/big_brains_and_early_mammals Lower Cretaceous mammal from China Jawbones of a Cretaceous marsupial from Mongolia Mammal fossil from the Cretaceous of Mongolia Reconstructed Cretaceous Mammal Early Cretaceous mammal ate small dinosaurs Repenomamus robustus fed on psittacosaurs. -

Paleontological Discoveries in the Chorrillo Formation (Upper Campanian-Lower Maastrichtian, Upper Cretaceous), Santa Cruz Province, Patagonia, Argentina
Rev. Mus. Argentino Cienc. Nat., n.s. 21(2): 217-293, 2019 ISSN 1514-5158 (impresa) ISSN 1853-0400 (en línea) Paleontological discoveries in the Chorrillo Formation (upper Campanian-lower Maastrichtian, Upper Cretaceous), Santa Cruz Province, Patagonia, Argentina Fernando. E. NOVAS1,2, Federico. L. AGNOLIN1,2,3, Sebastián ROZADILLA1,2, Alexis M. ARANCIAGA-ROLANDO1,2, Federico BRISSON-EGLI1,2, Matias J. MOTTA1,2, Mauricio CERRONI1,2, Martín D. EZCURRA2,5, Agustín G. MARTINELLI2,5, Julia S. D´ANGELO1,2, Gerardo ALVAREZ-HERRERA1, Adriel R. GENTIL1,2, Sergio BOGAN3, Nicolás R. CHIMENTO1,2, Jordi A. GARCÍA-MARSÀ1,2, Gastón LO COCO1,2, Sergio E. MIQUEL2,4, Fátima F. BRITO4, Ezequiel I. VERA2,6, 7, Valeria S. PEREZ LOINAZE2,6 , Mariela S. FERNÁNDEZ8 & Leonardo SALGADO2,9 1 Laboratorio de Anatomía Comparada y Evolución de los Vertebrados. Museo Argentino de Ciencias Naturales “Bernardino Rivadavia”, Avenida Ángel Gallardo 470, Buenos Aires C1405DJR, Argentina - fernovas@yahoo. com.ar. 2 Consejo Nacional de Investigaciones Científicas y Técnicas, Argentina. 3 Fundación de Historia Natural “Felix de Azara”, Universidad Maimonides, Hidalgo 775, C1405BDB Buenos Aires, Argentina. 4 Laboratorio de Malacología terrestre. División Invertebrados Museo Argentino de Ciencias Naturales “Bernardino Rivadavia”, Avenida Ángel Gallardo 470, Buenos Aires C1405DJR, Argentina. 5 Sección Paleontología de Vertebrados. Museo Argentino de Ciencias Naturales “Bernardino Rivadavia”, Avenida Ángel Gallardo 470, Buenos Aires C1405DJR, Argentina. 6 División Paleobotánica. Museo Argentino de Ciencias Naturales “Bernardino Rivadavia”, Avenida Ángel Gallardo 470, Buenos Aires C1405DJR, Argentina. 7 Área de Paleontología. Departamento de Geología, Universidad de Buenos Aires, Pabellón 2, Ciudad Universitaria (C1428EGA) Buenos Aires, Argentina. 8 Instituto de Investigaciones en Biodiversidad y Medioambiente (CONICET-INIBIOMA), Quintral 1250, 8400 San Carlos de Bariloche, Río Negro, Argentina. -

A New Mammaliaform from the Early Jurassic and Evolution Of
R EPORTS tary trough with a shelflike dorsal medial ridge, and all other nonmammalian mamma- A New Mammaliaform from the liaforms have a medial concavity on the man- dibular angle (8–14, 23), as in nonmamma- Early Jurassic and Evolution of liaform cynodonts (9, 14, 24–27). The post- dentary trough and the medial concavity on Mammalian Characteristics the mandibular angle respectively accommo- dated the prearticular/surangular and the re- Zhe-Xi Luo,1* Alfred W. Crompton,2 Ai-Lin Sun3 flected lamina of the angular (9, 25–27) that are the homologs to the mammalian middle A fossil from the Early Jurassic (Sinemurian, ϳ195 million years ago) represents ear bones (9, 14, 16–21, 23, 26). The absence a new lineage of mammaliaforms, the extinct groups more closely related to of these structures indicates that the postden- the living mammals than to nonmammaliaform cynodonts. It has an enlarged tary bones (“middle ear ossicles”) must have cranial cavity, but no postdentary trough on the mandible, indicating separation been separated from the mandible (Fig. 3). of the middle ear bones from the mandible. This extends the earliest record of Hadrocodium lacks the primitive meckelian these crucial mammalian features by some 45 million years and suggests that sulcus of the mandible typical of all nonmam- separation of the middle ear bones from the mandible and the expanded brain maliaform cynodonts (24–27), stem groups vault could be correlated. It shows that several key mammalian evolutionary of mammaliaforms (8, 9, 14, 23, 26, 27), innovations in the ear region, the temporomandibular joint, and the brain vault triconodontids (28, 29), and nontribosphenic evolved incrementally through mammaliaform evolution and long before the therian mammals (30). -

Mesozoic: the Dark Age for Mammals!
Ed’s Simplified History of the Mammals Note progression from Pelycosaurs (1) to Therapsids and Cynodonts (2) in Triassic. Stem mammals appeared in Late Triassic and Early Jurassic (3). Relationships among the Middle Jurassic forms (4) are controversial (see handout). Therian clade, identified by the tribosphenic molar (5), emerged at the end of the Jurassic, Early Cretaceous. A slightly more detailed version… in case you like something that looks more slick From Pough et al. 2009. Vertebrate Life, 8th ed. Pelycosaurs Dominated the late Permian, gave rise to therapsids Therapsids Rapid radiation in late Permian, around 270 MYA Still “mammal-like reptiles” The mass extinction at the end of the Permian was the greatest loss of diversity ever with >80% of all known genera and about 90% of all species going extinct, both terrestrial and marine. Cynodonts Late Permian to mid Triassic Last remaining group of therapsids, survived mass extinction at the end of the Permian. Persisted well Only 1 lineage of into Triassic and developed cynodonts survived many features associated through the late Triassic, with mammals. and this group became ancestors of mammals. Mesozoic: the Dark Age for Mammals! multituberculate Morganucodon, one of the earliest mammals (What else was happening in the Late Triassic and Jurassic Hadrocodium that may have contributed to mammals becoming small and Most were very small with nocturnal?) conservative morphology ...but new fossil finds indicate more diversity than we thought Repenomanus Still, largest known mammal during Mesozic Most were shrew to is no larger than a mouse sized, for 125 woodchuck million years! Some Mesozoic events and mammals you should know 1. -
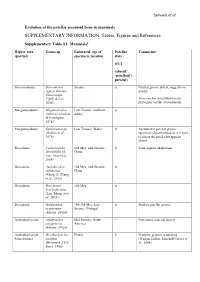
SUPPLEMENTARY INFORMATION: Tables, Figures and References
Samuels et al. Evolution of the patellar sesamoid bone in mammals SUPPLEMENTARY INFORMATION: Tables, Figures and References Supplementary Table S1: Mammals$ Higher taxa Genus sp. Estimated. age of Patellar Comments# (partial) specimen, location state 0/1/2 (absent/ ‘patelloid’/ present) Sinoconodonta Sinoconodon Jurassic 0 Patellar groove absent, suggests no rigneyi (Kielan- patella Jaworowska, Cifelli & Luo, Sinoconodon is included on our 2004) phylogeny within tritylodontids. Morganucodonta Megazostrodon Late Triassic, southern 0 rudnerae (Jenkins Africa & Parrington, 1976) Morganucodonta Eozostrodon sp. Late Triassic, Wales 0 Asymmetric patellar groove, (Jenkins et al., specimens disarticulated so it is hard 1976) to assess the patella but appears absent Docodonta Castorocauda 164 Mya, mid-Jurassic, 0 Semi-aquatic adaptations lutrasimilis (Ji, China Luo, Yuan et al., 2006) Docodonta Agilodocodon 164 Mya, mid-Jurassic, 0 scansorius China (Meng, Ji, Zhang et al., 2015) Docodonta Docofossor 160 Mya 0 brachydactylus (Luo, Meng, Ji et al., 2015) Docodonta Haldanodon 150-155 Mya, Late 0 Shallow patellar groove exspectatus Jurassic, Portugal (Martin, 2005b) Australosphenida Asfaltomylos Mid-Jurassic, South ? Postcranial material absent patagonicus America (Martin, 2005a) Australosphenida Ornithorhynchus Extant 2 Platypus, genome sequenced Monotremata anatinus (Warren, Hillier, Marshall Graves et (Herzmark, 1938; al., 2008) Rowe, 1988) Samuels et al. Australosphenida Tachyglossus + Extant 2 Echidnas Monotremata Zaglossus spp. (Herzmark, 1938; Rowe, 1988) Mammaliaformes Fruitafossor 150 Mya, Late Jurassic, 0 Phylogenetic status uncertain indet. windscheffeli (Luo Colorado & Wible, 2005) Mammaliaformes Volaticotherium Late Jurassic/Early ? Hindlimb material incomplete indet. antiquus (Meng, Cretaceous Hu, Wang et al., 2006) Eutriconodonta Jeholodens 120-125 Mya, Early 0 Poorly developed patellar groove jenkinsi (Ji, Luo Cretaceous, China & Ji, 1999) Eutriconodonta Gobiconodon spp. -

Two New Species of Gobiconodon (Mammalia, Eutriconodonta, Gobiconodontidae) from the Lower Cretaceous Shahai and Fuxin Formations, Northeastern China
Historical Biology An International Journal of Paleobiology ISSN: 0891-2963 (Print) 1029-2381 (Online) Journal homepage: http://www.tandfonline.com/loi/ghbi20 Two new species of Gobiconodon (Mammalia, Eutriconodonta, Gobiconodontidae) from the Lower Cretaceous Shahai and Fuxin formations, northeastern China Nao Kusuhashi, Yuan-Qing Wang, Chuan-Kui Li & Xun Jin To cite this article: Nao Kusuhashi, Yuan-Qing Wang, Chuan-Kui Li & Xun Jin (2016) Two new species of Gobiconodon (Mammalia, Eutriconodonta, Gobiconodontidae) from the Lower Cretaceous Shahai and Fuxin formations, northeastern China, Historical Biology, 28:1-2, 14-26 To link to this article: http://dx.doi.org/10.1080/08912963.2014.977881 Published online: 01 Oct 2015. Submit your article to this journal View related articles View Crossmark data Full Terms & Conditions of access and use can be found at http://www.tandfonline.com/action/journalInformation?journalCode=ghbi20 Download by: [University of Sussex Library] Date: 01 October 2015, At: 18:24 Historical Biology, 2016 Vol. 28, Nos. 1–2, 14–26, http://dx.doi.org/10.1080/08912963.2014.977881 Two new species of Gobiconodon (Mammalia, Eutriconodonta, Gobiconodontidae) from the Lower Cretaceous Shahai and Fuxin formations, northeastern China Nao Kusuhashia*, Yuan-Qing Wangb, Chuan-Kui Lib and Xun Jinb aDepartment of Earth’s Evolution and Environment, Graduate School of Science and Engineering, Ehime University, Ehime 790-8577, Japan; bKey Laboratory of Vertebrate Evolution and Human Origins of Chinese Academy of Sciences, Institute of Vertebrate Paleontology and Paleoanthropology, Chinese Academy of Sciences, Beijing 100044, P.R. China (Received 29 July 2014; accepted 14 October 2014) Two new gobiconodontid mammals, Gobiconodon tomidai sp. -

Science Journals
RESEARCH | REPORT PALEONTOLOGY the hyoid apparatus in monotremes and placen- tals, and in a modified condition in marsupials (for details, see the supplementary materials) New Jurassic mammaliaform (16–18). Based on the distinctly mammal-like morphol- sheds light on early evolution ogy of the hyoid elements of Microdocodon,we can now identify hyoid elements of several other mammaliaforms (16)(figs.S4toS9).TheJurassic of mammal-like hyoid bones haramiyidan Vilevolodon has preserved basihyal 1,2 3 4 and ceratohyal bones (fig. S6 and movie S2) (19). Chang-Fu Zhou *, Bhart-Anjan S. Bhullar *, April I. Neander , The Cretaceous eutriconodontan Yanoconodon 5† 4† Thomas Martin , Zhe-Xi Luo also has preserved hyoid elements (fig. S6) (20). Computed tomography (CT) scanning has re- We report a new Jurassic docodontan mammaliaform found in China that is preserved vealed the basihyal, thyrohyals, ceratohyals, and with the hyoid bones. Its basihyal, ceratohyal, epihyal, and thyrohyal bones have mobile epihyals of the trechnotherian Maotherium and joints and are arranged in a saddle-shaped configuration, as in the mobile linkage the multituberculate Sinobaatar of the Cretaceous of the hyoid apparatus of extant mammals. These are fundamentally different from (figs. S7 and S8 and movie S2) (16). Addition- the simple hyoid rods of nonmammaliaform cynodonts, which were likely associated ally, Sinobaatar has preserved stylohyal bones with a wide, nonmuscularized throat, as seen in extant reptiles. The hyoid apparatus (fig. S7), which is similar to the Cretaceous multi- provides a framework for the larynx and for the constricted, muscularized esophagus, tuberculate Kryptobaatar (21). We interpret this crucial for transport and powered swallowing of the masticated food and liquid in extant to mean that multituberculates have an integro- Downloaded from mammals.