Jaw Roll and Jaw Yaw in Early Mammals
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Versión Disponible En PDF
nicolás r chimento, Federico L agnolin y Fernando e novas Museo Argentino de Ciencias Naturales Bernardino Rivadavia Necrolestes un mamífero patagónico que sobrevivió a la extinción de los dinosaurios Un descubrimiento patagónico desembocadura del río Santa Cruz, descubrieron esque- letos fósiles prácticamente completos del Necrolestes. El es- En 1891, Florentino Ameghino (1854-1911) dio a tudio de esos esqueletos llevó a pensar que se trataba de conocer unos restos fósiles encontrados por su hermano un mamífero muy arcaico en la historia de la evolución, Carlos (1865-1936) en las barrancas de Monte Obser- más que un ancestro de los topos, como había supuesto vación, en la provincia de Santa Cruz, en yacimientos Ameghino. Por determinados rasgos se pensó que podía de unos 17 millones de años de antigüedad. Determinó haber sido un marsupial, es decir, un pariente lejano de las que pertenecían a un pequeño –escasos 10cm de largo, comadrejas, los canguros y los coalas actuales. del hocico a la cola– y desconocido mamífero extin- Ciertos investigadores aceptaron esta última hipótesis guido. Estudió los diminutos huesos y consideró que de parentesco, pero otros se mostraron escépticos acerca el animal habría sido un pariente lejano de los topos de ella y se inclinaron por considerar inciertas las rela- africanos vivientes. Le dio el nombre científicoN ecrolestes ciones genealógicas del diminuto mamífero. Así, su po- patagonensis, es decir, ladrón de tumbas de la Patagonia, en sición en el árbol evolutivo de los mamíferos fue objeto alusión a sus hábitos excavadores. El hallazgo, publica- de debate durante gran parte del siglo XX. Para algunos, do por Ameghino en el número de la Revista Argentina de era pariente lejano de las mulitas; otros seguían pensan- Historia Natural citado entre las lecturas sugeridas, atrajo do que podía estar relacionado con los topos, y para un la atención del ámbito científico, ya que hasta ese mo- tercer grupo, sus vínculos eran con los marsupiales aus- mento en Sudamérica no se habían encontrado restos tralianos. -

Aptian–Albian) of Texas and Oklahoma
Reappraisal of the tribosphenidan mammals from the Trinity Group (Aptian–Albian) of Texas and Oklahoma BRIAN M. DAVIS and RICHARD L. CIFELLI Davis, B.M. and Cifelli, R.L. 2011. Reappraisal of the tribosphenidan mammals from the Trinity Group (Aptian–Albian) of Texas and Oklahoma. Acta Palaeontologica Polonica 56 (3): 441–462. The Trinity therians have long been the focus of attempts to reconstruct the evolutionary history of higher mammals, es− pecially in the context of the development of tribospheny. In this paper, we update the taxonomy of the tribosphenidan taxa known from the Trinity Group and establish with more confidence the premolar/molar count in each. Many isolated specimens can be referred to a specific tooth locus. Additional diversity is revealed within the Deltatheroida, with the de− scription of an additional species of Oklatheridium; Pappotherium is here considered a likely metatherian based on the in− ferred presence of four molars, while Holoclemensia is a basal eutherian (the opposite of some traditional interpretations). The remainder of the genera, Kermackia and Slaughteria, cannot be allied with either of the living groups of tribo− sphenidan mammals using the available data. We identify strong morphological diversity within this assemblage of stem taxa, including modifications to the traditional tribosphenic occlusal pattern in Kermackia. Mammalian evolution at the base of the tribosphenidan radiation was complex, and this underscores the need for caution when interpreting the mor− phology and relationships of taxa known by incomplete material. Key words: Tribosphenida, Metatheria, Eutheria, Deltatheroida, Trinity Group, Early Cretaceous. Brian M. Davis [[email protected]] and Richard L. Cifelli [[email protected]], Department of Zoology and Sam Noble Oklahoma Museum of Natural History, University of Oklahoma, 2401 Chautauqua Ave, Norman, OK, 73072, USA. -

Recent Advances in Studies on Mesozoic and Paleogene Mammals in China
Vol.24 No.2 2010 Paleomammalogy Recent Advances in Studies on Mesozoic and Paleogene Mammals in China WANG Yuanqing* and NI Xijun Institute of Vertebrate Paleontology and Paleoanthropology, CAS, Beijing 10004, China ike in other fields of paleontology, research in from the articular of the lower jaw and the quadrate of the paleomammalogy mainly falls into two aspects. cranium following the process of reduction and detachment LOne is related to the biological nature of fossil from the reptilian mandible, are new elements of the bony mammals, such as their systematics, origin, evolution, chain in the mammalian middle ear. The appearance of phylogenetic relationships, transformation of key features mammalian middle ear allows mammals to hear the sound and paleobiogeography, and the other is related to the of higher frequencies than reptiles do. Generally speaking, geological context, involving biostratigraphy, biochronology, a widely accepted hypothesis is that mammals originated faunal turnover and its relations to the global and regional from a certain extinct reptilian group. The formation and environmental changes. In the last decade, a number of development of the definitive mammalian middle ear well-preserved mammalian specimens were collected from (DMME) has thus become one of the key issues in the study different localities around the country. Such discoveries have of mammalian evolution and has drawn the attention from provided significant information for understanding the early many researchers for many years. evolution of mammals and reconstructing the phylogeny of Developmental biological studies have proven the early mammals. function of the Meckel’s cartilage and its relationship to Studies of Chinese Mesozoic mammals achieved the ear ossicles during the development of mammalian remarkable progress in the past several years. -

Mammals from the Mesozoic of Mongolia
Mammals from the Mesozoic of Mongolia Introduction and Simpson (1926) dcscrihed these as placental (eutherian) insectivores. 'l'he deltathcroids originally Mongolia produces one of the world's most extraordi- included with the insectivores, more recently have narily preserved assemblages of hlesozoic ma~nmals. t)een assigned to the Metatheria (Kielan-Jaworowska Unlike fossils at most Mesozoic sites, Inany of these and Nesov, 1990). For ahout 40 years these were the remains are skulls, and in some cases these are asso- only Mesozoic ~nanimalsknown from Mongolia. ciated with postcranial skeletons. Ry contrast, 'I'he next discoveries in Mongolia were made by the Mesozoic mammals at well-known sites in North Polish-Mongolian Palaeontological Expeditions America and other continents have produced less (1963-1971) initially led by Naydin Dovchin, then by complete material, usually incomplete jaws with den- Rinchen Barsbold on the Mongolian side, and Zofia titions, or isolated teeth. In addition to the rich Kielan-Jaworowska on the Polish side, Kazi~nierz samples of skulls and skeletons representing Late Koualski led the expedition in 1964. Late Cretaceous Cretaceous mam~nals,certain localities in Mongolia ma~nmalswere collected in three Gohi Desert regions: are also known for less well preserved, but important, Bayan Zag (Djadokhta Formation), Nenlegt and remains of Early Cretaceous mammals. The mammals Khulsan in the Nemegt Valley (Baruungoyot from hoth Early and Late Cretaceous intervals have Formation), and llcrmiin 'ISav, south-\vest of the increased our understanding of diversification and Neniegt Valley, in the Red beds of Hermiin 'rsav, morphologic variation in archaic mammals. which have heen regarded as a stratigraphic ecluivalent Potentially this new information has hearing on the of the Baruungoyot Formation (Gradzinslti r't crl., phylogenetic relationships among major branches of 1977). -

Mesozoic: the Dark Age for Mammals!
Ed’s Simplified History of the Mammals Note progression from Pelycosaurs (1) to Therapsids and Cynodonts (2) in Triassic. Stem mammals appeared in Late Triassic and Early Jurassic (3). Relationships among the Middle Jurassic forms (4) are controversial (see handout). Therian clade, identified by the tribosphenic molar (5), emerged at the end of the Jurassic, Early Cretaceous. A slightly more detailed version… in case you like something that looks more slick From Pough et al. 2009. Vertebrate Life, 8th ed. Pelycosaurs Dominated the late Permian, gave rise to therapsids Therapsids Rapid radiation in late Permian, around 270 MYA Still “mammal-like reptiles” The mass extinction at the end of the Permian was the greatest loss of diversity ever with >80% of all known genera and about 90% of all species going extinct, both terrestrial and marine. Cynodonts Late Permian to mid Triassic Last remaining group of therapsids, survived mass extinction at the end of the Permian. Persisted well Only 1 lineage of into Triassic and developed cynodonts survived many features associated through the late Triassic, with mammals. and this group became ancestors of mammals. Mesozoic: the Dark Age for Mammals! multituberculate Morganucodon, one of the earliest mammals (What else was happening in the Late Triassic and Jurassic Hadrocodium that may have contributed to mammals becoming small and Most were very small with nocturnal?) conservative morphology ...but new fossil finds indicate more diversity than we thought Repenomanus Still, largest known mammal during Mesozic Most were shrew to is no larger than a mouse sized, for 125 woodchuck million years! Some Mesozoic events and mammals you should know 1. -
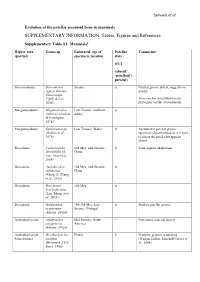
SUPPLEMENTARY INFORMATION: Tables, Figures and References
Samuels et al. Evolution of the patellar sesamoid bone in mammals SUPPLEMENTARY INFORMATION: Tables, Figures and References Supplementary Table S1: Mammals$ Higher taxa Genus sp. Estimated. age of Patellar Comments# (partial) specimen, location state 0/1/2 (absent/ ‘patelloid’/ present) Sinoconodonta Sinoconodon Jurassic 0 Patellar groove absent, suggests no rigneyi (Kielan- patella Jaworowska, Cifelli & Luo, Sinoconodon is included on our 2004) phylogeny within tritylodontids. Morganucodonta Megazostrodon Late Triassic, southern 0 rudnerae (Jenkins Africa & Parrington, 1976) Morganucodonta Eozostrodon sp. Late Triassic, Wales 0 Asymmetric patellar groove, (Jenkins et al., specimens disarticulated so it is hard 1976) to assess the patella but appears absent Docodonta Castorocauda 164 Mya, mid-Jurassic, 0 Semi-aquatic adaptations lutrasimilis (Ji, China Luo, Yuan et al., 2006) Docodonta Agilodocodon 164 Mya, mid-Jurassic, 0 scansorius China (Meng, Ji, Zhang et al., 2015) Docodonta Docofossor 160 Mya 0 brachydactylus (Luo, Meng, Ji et al., 2015) Docodonta Haldanodon 150-155 Mya, Late 0 Shallow patellar groove exspectatus Jurassic, Portugal (Martin, 2005b) Australosphenida Asfaltomylos Mid-Jurassic, South ? Postcranial material absent patagonicus America (Martin, 2005a) Australosphenida Ornithorhynchus Extant 2 Platypus, genome sequenced Monotremata anatinus (Warren, Hillier, Marshall Graves et (Herzmark, 1938; al., 2008) Rowe, 1988) Samuels et al. Australosphenida Tachyglossus + Extant 2 Echidnas Monotremata Zaglossus spp. (Herzmark, 1938; Rowe, 1988) Mammaliaformes Fruitafossor 150 Mya, Late Jurassic, 0 Phylogenetic status uncertain indet. windscheffeli (Luo Colorado & Wible, 2005) Mammaliaformes Volaticotherium Late Jurassic/Early ? Hindlimb material incomplete indet. antiquus (Meng, Cretaceous Hu, Wang et al., 2006) Eutriconodonta Jeholodens 120-125 Mya, Early 0 Poorly developed patellar groove jenkinsi (Ji, Luo Cretaceous, China & Ji, 1999) Eutriconodonta Gobiconodon spp. -

Palaeontologia Electronica CT Reconstructions and Relationships Of
Palaeontologia Electronica http://palaeo-electronica.org CT reconstructions and relationships of the Early Cretaceous tribosphenidan mammal, Slaughteria eruptens (Trinity Group Texas, USA) Dale A. Winkler, Louis L. Jacobs, Y. Kobayashi, and Michael J. Polcyn ABSTRACT Among the nine taxa of tribosphenidan (boreosphenidan) mammals named from the Early Cretaceous Trinity Group of Texas and Oklahoma, Slaughteria eruptens pro- vides unique information about the evolution of tooth replacement. High-resolution CT scans and SEM imagery elucidate the jaw anatomy of S. eruptens. Associated upper and lower dentitions from Asian Cretaceous mammals provide the basis for a statistical model to quantify the tooth size relationships of the often cited Trinity Group mammals Pappotherium pattersoni and Holoclemensia texana, which were named based upon upper molar teeth. Two groups of Trinity mammals are evident based on lower molar size. Most large teeth are referable to H. texana. The lower m1 of S. eruptens fits neatly into the size range predicted for lower molars of P. pattersoni, and it shares com- patible tooth cusp relationships. Slaughteria eruptens is regarded most parsimoniously as a junior synonym of P. pattersoni. Pappotherium pattersoni thus shares the alternate premolar replacement pattern of more primitive therian mammals and basal eutheri- ans, but lacks any hint of a submolariform last premolar, as typifies Eutheria. Other small therian mammal teeth from the Trinity Group should be evaluated as possible deciduous teeth. Dale A. Winkler. Department of Earth Sciences, Southern Methodist University, Dallas, TX 75275 USA. [email protected] Louis L. Jacobs. Department of Earth Sciences, Southern Methodist University, Dallas, TX 75275 USA. [email protected] Yoshi Kobayashi. -

Science Journals
RESEARCH | REPORT PALEONTOLOGY the hyoid apparatus in monotremes and placen- tals, and in a modified condition in marsupials (for details, see the supplementary materials) New Jurassic mammaliaform (16–18). Based on the distinctly mammal-like morphol- sheds light on early evolution ogy of the hyoid elements of Microdocodon,we can now identify hyoid elements of several other mammaliaforms (16)(figs.S4toS9).TheJurassic of mammal-like hyoid bones haramiyidan Vilevolodon has preserved basihyal 1,2 3 4 and ceratohyal bones (fig. S6 and movie S2) (19). Chang-Fu Zhou *, Bhart-Anjan S. Bhullar *, April I. Neander , The Cretaceous eutriconodontan Yanoconodon 5† 4† Thomas Martin , Zhe-Xi Luo also has preserved hyoid elements (fig. S6) (20). Computed tomography (CT) scanning has re- We report a new Jurassic docodontan mammaliaform found in China that is preserved vealed the basihyal, thyrohyals, ceratohyals, and with the hyoid bones. Its basihyal, ceratohyal, epihyal, and thyrohyal bones have mobile epihyals of the trechnotherian Maotherium and joints and are arranged in a saddle-shaped configuration, as in the mobile linkage the multituberculate Sinobaatar of the Cretaceous of the hyoid apparatus of extant mammals. These are fundamentally different from (figs. S7 and S8 and movie S2) (16). Addition- the simple hyoid rods of nonmammaliaform cynodonts, which were likely associated ally, Sinobaatar has preserved stylohyal bones with a wide, nonmuscularized throat, as seen in extant reptiles. The hyoid apparatus (fig. S7), which is similar to the Cretaceous multi- provides a framework for the larynx and for the constricted, muscularized esophagus, tuberculate Kryptobaatar (21). We interpret this crucial for transport and powered swallowing of the masticated food and liquid in extant to mean that multituberculates have an integro- Downloaded from mammals. -

How Many Kingdoms of Life? Eukaryotic Phylogeny and Philosophy of Systematics
How many kingdoms of life? Eukaryotic phylogeny and philosophy of systematics Łukasz Lamża Copernicus Center for Interdisciplinary Studies, Jagiellonian University Abstract According to contemporary understanding of the universal tree of life, the traditionally recognized kingdoms of eukaryotic organisms— Protista, Fungi, Animalia and Plantae—are irregularly interspersed in a vast phylogenetic tree. There are numerous groups that in any Linnaean classification advised by phylogenetic relationships (i.e. a Hennigian system) would form sister groups to those kingdoms, therefore requiring us to admit them the same rank. In practice, this would lead to the creation of ca. 25-30 new kingdoms that would now be listed among animals and plants as “major types of life”. This poses problems of an aesthetic and educational nature. There are, broadly speaking, two ways to deal with that issue: a) ignore the aesthetic and educational arguments and propose classification sys- tems that are fully consistent with the Hennigian principles of phylo- genetic classification, i.e. are only composed of monophyletic taxa; CC-BY-NC-ND 4.0 b) ignore Hennigian principles and bunch small, relatively uncharac- • teristic groups into paraphyletic taxa, creating systems that are more 227 – convenient. In the paper, I present the debate and analyze the pros 203 and cons of both options, briefly commenting on the deeper, third resolution, which would be to abandon classification systems entirely. Philosophical Problems in Science (Zagadnienia FilozoficzneNo w Nauce) 66 (2019), pp. 204 Łukasz Lamża Recent advances in eukaryotic classification and phylogeny are com- mented in the light of the philosophical question of the purpose and design principles of biological classification systems. -

Mammalian Fauna of the Late Jurassic Guimarota Ecosystem
Asociación Paleontológica Argentina. Publicación Especial 7 ISSN 0328-347X VII International Symposium on Mesozoic Terrestrial Ecosystems: 123-126. Buenos Aires, 30-6-2001 MaMMalian fauna of the Late Jurassic Guimarota ecoSyStem Thomas MARTIN1 Abstract. The Late Jurassic (Kirnmeridgian) Guimarota loca lit y near Leiria in west-central Portugal has yielded more than 800 mammalian dentaries and partial skulls representing the largest sample of Late Jurassic mammals in the world. However, despite the enormous number of specimens collected, the mam- malian fauna appears somewhat depauperate. So far, only Multituberculata (several genera of Paulchoffatiidae; 28% of total number), Docodonta (Halda nadan exspectaius Kühne and Krusat; 24%), and Holotheria (48%) represented by Paurodontidae (Henkelatherium guimaratae Krebs and Drescheratherium acutum Krebs) and Dryolestidae (Dryalestes leiriensis Martín, Krebsoiherium Iusiianicum Martin, and Guimaroiodus inflatus Martin) have been detected. "Triconodonta" and Syrnmetrodonta, which are well represented at other Late Jurassic localities (e.g., Morrison Forrnation), are missing. To recover the rare mammalian taxa of the Guimarota ecosystem, a project was initiated to study the nearly 7000 isolated mammalian teeth that had been obtained by screenwashing. With a single exception, the same taxa are represented. Among the thousands of isolated teeth, however, 25 lower and 23 upper premolars and mo- lars of a tiny primitive Zatheria have been found, of which the lower molars closely resemble the "Porto Pinheiro molar". After sorting the isolated teeth, the mammalian fauna of the Guimarota ecosystem prob- ably is completely recorded. Apparently, the coastal swamp in the Lusitanian graben where the lignite formed represented a stressed environment not appropriate for some marnmalian groups. -

Dual Origin of Tribosphenic Mammals
articles Dual origin of tribosphenic mammals Zhe-Xi Luo*, Richard L. Cifelli² & Zo®a Kielan-Jaworowska³ *Section of Vertebrate Paleontology, Carnegie Museum of Natural History, Pittsburgh, Pennsylvania 15213, USA ² Oklahoma Museum of Natural History, 2401 Chautauqua, Norman, Oklahoma 73072, USA ³ Institute of Paleobiology, Polish Academy of Sciences, ulica Twarda 51/55, PL-00-818 Warszawa, Poland ............................................................................................................................................................................................................................................................................ Marsupials, placentals and their close therian relatives possess complex (tribosphenic) molars that are capable of versatile occlusal functions. This functional complex is widely thought to be a key to the early diversi®cation and evolutionary success of extant therians and their close relatives (tribosphenidans). Long thought to have arisen on northern continents, tribosphenic mammals have recently been reported from southern landmasses. The great age and advanced morphology of these new mammals has led to the alternative suggestion of a Gondwanan origin for the group. Implicit in both biogeographic hypotheses is the assumption that tribosphenic molars evolved only once in mammalian evolutionary history. Phylogenetic and morphometric analyses including these newly discovered taxa suggest a different interpretation: that mammals with tribosphenic molars are not monophyletic. Tribosphenic -

Aspects of the Microvertebrate Fauna of the Early Cretaceous (Barremian) Wessex Formation of the Isle of Wight, Southern England
ASPECTS OF THE MICROVERTEBRATE FAUNA OF THE EARLY CRETACEOUS (BARREMIAN) WESSEX FORMATION OF THE ISLE OF WIGHT, SOUTHERN ENGLAND By STEVEN CHARLES SWEETMAN M.A. (Oxon.) 1980 F.G.S. A thesis submitted in partial fulfilment of the requirements for the award of the degree of Doctor of Philosophy of the University of Portsmouth School of Earth and Environmental Sciences, University of Portsmouth, Burnaby Building, Burnaby Road, Portsmouth, PO1 3QL, U.K. April, 2007 0 Disclaimer Whilst registered for this degree, I have not registered for any other award. No part of this work has been submitted for any other academic award. 1 Acknowledgements At inception of this project there was a significant risk that the Wessex Formation would not yield a microvertebrate fauna. I would, therefore, like to express special thanks to Dave Martill (University of Portsmouth) for his initial support and for securing the research scholarship which made this study possible. I would also like to thank him for his supervision, generous support, encouragement and advice thereafter. Special thanks also to Susan Evans (UCL) for her enthusiastic help and advice on all matters relating to microvertebrates in general, and lizards in particular, and to Jerry Hooker (NHM) for everything relating to mammals; also to Brian Gasson for his support in the field and for the generous donation of many exceptional specimens from his private collection. The broad scope of this study has engendered the help, support and advice of many others and I am grateful to all. At the University