Nose-To-Brain Delivery
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Perception of Facial Expressions in Social Anxiety and Gaze Anxiety
The Pegasus Review: UCF Undergraduate Research Journal (URJ) Volume 9 Issue 1 Article 6 2016 Perception of Facial Expressions in Social Anxiety and Gaze Anxiety Aaron Necaise University of Central Florida, [email protected] Part of the Psychology Commons Find similar works at: https://stars.library.ucf.edu/urj University of Central Florida Libraries http://library.ucf.edu This Article is brought to you for free and open access by the Office of Undergraduate Research at STARS. It has been accepted for inclusion in The Pegasus Review: UCF Undergraduate Research Journal (URJ) by an authorized editor of STARS. For more information, please contact [email protected]. Recommended Citation Necaise, Aaron (2016) "Perception of Facial Expressions in Social Anxiety and Gaze Anxiety," The Pegasus Review: UCF Undergraduate Research Journal (URJ): Vol. 9 : Iss. 1 , Article 6. Available at: https://stars.library.ucf.edu/urj/vol9/iss1/6 Necaise: Perception of Facial Expressions Social Anxiety & Gaze Anxiety Published Vol. 9.1: 40-47 October 19th, 2017 THE UNIVERSITY OF CENTRAL FLORIDA UNDERGRADUATE RESEARCH JOURNAL Analysis of the Pathomechanism and Treatment of Migraines Related to the Role of the Neuropeptide CGRP By: Marvi S. Qureshi Faculty Mentor: Dr. Mohtashem Samsam UCF Burnett School of Biomedical Sciences ABSTRACT: Migraines are a type of headache that specifically act on only one side of the head, although about 30% of patients with migraines may experience a bilateral headache. Migraines are brain disorders that typically involve issues of sensory processing taking place in the brainstem. Possible causation has been linked to blood vessels, blood flow, and oxygen levels in the brain. -

Clinical Review Nursingingeneralpractice
The health benefits of nose breathing Item Type Article Authors Allen, Ruth Publisher Nursing in General Practice Journal Nursing in General Practice Download date 01/10/2021 07:15:20 Link to Item http://hdl.handle.net/10147/559021 Find this and similar works at - http://www.lenus.ie/hse clinical review nursingingeneralpractice The health benefits of nose breathing DR Alan RUth, BehaviouRal Medicine PRactitioneR “For breath is life, and if you breathe well you will live long on earth.” sanskrit Proverb For the most part people are unaware of their breathing and take it for granted that they do it correctly. t has been estimated that approximately one third of people ing. However, it has been estimated that up to 30-50% of modern don’t breathe well enough to sustain normal health. These adults breathe through the mouth, especially during the early people do not get enough oxygenation of their cells, tissues morning hours. and organs. In the book Behavioural and Psychological Ap- Mouth breathing is common in individuals whose nasal proaches to Breathing Disorders, Dr Chandra Patel describes passages are blocked or restricted. A deviated nasal septum Ithe problem with breathing as follows: or small nostril size can lead a person to breathe through their “We start life with a breath, and the process continues mouth instead of their nose. However, breathing through the automatically for the rest of our lives. Because breathing mouth most of the time was not nature’s intention. Many studies continues on its own, without our awareness, it does not have demonstrated that chronic mouth breathing can result in a necessarily mean that it is always functioning for optimum number of adverse health consequences (see Table 1). -

Potential Mechanisms of Prospective Antimigraine Drugs: a Focus on Vascular (Side) Effects
CORE Metadata, citation and similar papers at core.ac.uk Provided by Elsevier - Publisher Connector Pharmacology & Therapeutics 129 (2011) 332–351 Contents lists available at ScienceDirect Pharmacology & Therapeutics journal homepage: www.elsevier.com/locate/pharmthera Associate Editor: John Fozard Potential mechanisms of prospective antimigraine drugs: A focus on vascular (side) effects Kayi Y. Chan a, Steve Vermeersch b, Jan de Hoon b, Carlos M. Villalón c, Antoinette MaassenVanDenBrink a,⁎ a Division of Vascular Medicine and Pharmacology, Department of Internal Medicine, Erasmus Medical Center, P.O. Box 2040, 3000 CA Rotterdam, The Netherlands b Center for Clinical Pharmacology, University Hospitals Leuven, Campus Gasthuisberg, (K.U. Leuven), Leuven, Belgium c Departamento de Farmacobiología, Cinvestav-Coapa, Czda. de los Tenorios 235, Col. Granjas-Coapa, Deleg. Tlalpan, C.P. 14330, México D.F., Mexico article info abstract Available online 2 December 2010 Currently available drugs for the acute treatment of migraine, i.e. ergot alkaloids and triptans, are cranial vasoconstrictors. Although cranial vasoconstriction is likely to mediate—at least a part of—their therapeutic Keywords: effects, this property also causes vascular side-effects. Indeed, the ergot alkaloids and the triptans have been Antimigraine drugs reported to induce myocardial ischemia and stroke, albeit in extremely rare cases, and are contraindicated in Neuropeptides patients with known cardiovascular risk factors. In view of these limitations, novel antimigraine drugs -

Current and Prospective Pharmacological Targets in Relation to Antimigraine Action
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Erasmus University Digital Repository Naunyn-Schmiedeberg’s Arch Pharmacol (2008) 378:371–394 DOI 10.1007/s00210-008-0322-7 REVIEW Current and prospective pharmacological targets in relation to antimigraine action Suneet Mehrotra & Saurabh Gupta & Kayi Y. Chan & Carlos M. Villalón & David Centurión & Pramod R. Saxena & Antoinette MaassenVanDenBrink Received: 8 January 2008 /Accepted: 6 June 2008 /Published online: 15 July 2008 # The Author(s) 2008 Abstract Migraine is a recurrent incapacitating neuro- (CGRP1 and CGRP2), adenosine (A1,A2,andA3), glutamate vascular disorder characterized by unilateral and throbbing (NMDA, AMPA, kainate, and metabotropic), dopamine, headaches associated with photophobia, phonophobia, endothelin, and female hormone (estrogen and progesterone) nausea, and vomiting. Current specific drugs used in the receptors. In addition, we have considered some other acute treatment of migraine interact with vascular receptors, targets, including gamma-aminobutyric acid, angiotensin, a fact that has raised concerns about their cardiovascular bradykinin, histamine, and ionotropic receptors, in relation to safety. In the past, α-adrenoceptor agonists (ergotamine, antimigraine therapy. Finally, the cardiovascular safety of dihydroergotamine, isometheptene) were used. The last two current and prospective antimigraine therapies is touched decades have witnessed the advent of 5-HT1B/1D receptor upon. agonists (sumatriptan and second-generation triptans), which have a well-established efficacy in the acute Keywords 5-HT. Antimigraine drugs . CGRP. treatment of migraine. Moreover, current prophylactic Noradrenaline . Migraine . Receptors treatments of migraine include 5-HT2 receptor antagonists, Ca2+ channel blockers, and β-adrenoceptor antagonists. Despite the progress in migraine research and in view of its Introduction complex etiology, this disease still remains underdiagnosed, and available therapies are underused. -

Serotonin Receptor Knockouts: a Moody Subject David Julius* Department of Cellular and Molecular Pharmacology, University of California, San Francisco, CA 94143-0450
Proc. Natl. Acad. Sci. USA Vol. 95, pp. 15153–15154, December 1998 Commentary Serotonin receptor knockouts: A moody subject David Julius* Department of Cellular and Molecular Pharmacology, University of California, San Francisco, CA 94143-0450 The neurotransmitter serotonin (5-hydroxytryptamine; 5-HT) receptors are expressed in a number of brain regions to which is believed to play a significant role in determining one’s serotonergic neurons project, including the hippocampus, ce- emotional state. Indeed, serotonergic synapses are sites of rebral cortex, and amygdala (11, 12). As in the case of action for a number of mood-altering drugs, including the presynaptic autoreceptors, activation of postsynaptic 5-HT1A now-legendary antidepressant Prozac (fluoxetine) (1). As a receptors leads to hyperpolarization of the neuron and the result, there has been tremendous interest in identifying consequent inhibition of neurotransmitter release. This effect molecular components of the serotonergic system, including appears to be mediated through a biochemical signaling path- cell surface receptors and transporters, and understanding way in which 5-HT1A receptors activate a G protein (Gi)- whether and how these proteins contribute to the regulation of coupled inwardly rectifying potassium channel (13, 14). mood and emotion. This quest is driven, in part, by the In light of the pharmacological evidence that 5-HT1A re- possibility that behavioral disorders, such as depression or ceptors exert negative ‘‘feedback’’ control on serotonergic anxiety, may be linked to deficits in one or more components neurons, one would predict that mice lacking this receptor of this signaling system. Such information could, in turn, focus should show elevated levels of extraneuronal serotonin, or an attention on specific targets for the development of novel increase in the amount of serotonin released after nerve drugs with which to treat psychiatric disorders. -

Does Sumatriptan Cross the Blood–Brain Barrier in Animals and Man?
J Headache Pain (2010) 11:5–12 DOI 10.1007/s10194-009-0170-y REVIEW ARTICLE Does sumatriptan cross the blood–brain barrier in animals and man? Peer Carsten Tfelt-Hansen Received: 24 August 2009 / Accepted: 27 October 2009 / Published online: 10 December 2009 Ó Springer-Verlag 2009 Abstract Sumatriptan, a relatively hydrophilic triptan, development [6, 7] or an effect on trigeminovascular nerves based on several animal studies has been regarded to be [6]. A peripheral effect on trigeminal vascular nerves was unable to cross the blood–brain barrier (BBB). In more indicated by the blocking effect of sumatriptan of neuro- recent animal studies there are strong indications that genically mediated plasma extravasation [8]. Inhibitors of sumatriptan to some extent can cross the BBB. The CNS neurogenic inflammation (NI) were, however, ineffective in adverse events of sumatriptan in migraine patients and the treatment of migraine [9] and it is thus difficult to normal volunteers also indicate a more general effect of ascribe a pivotal role for NI in migraine. In 1996 it was, sumatriptan on CNS indicating that the drug can cross the based on the effect of zolmitriptan, suggested that inhibition BBB in man. It has been discussed whether a defect in the of trigeminal neurons in the brain stem by lipophilic triptans BBB during migraine attacks could be responsible for a may play a role in the anti-migraine effect of these drugs possible central effect of sumatriptan in migraine. This and that these results offered the prospect of a third path- review suggests that there is no need for a breakdown in the ophysiological target site for triptans [10]. -

Histamine and Antihistaminics Chapter 11
Histamine and Antihistaminics Chapter 11 HISTAMINE Histamine, meaning ‘tissue amine’ (histos—tissue) is almost ubiquitously present in animal tissues and in certain plants, e.g. stinging nettle. Its pharmacology was studied in detail by Dale in the beginning of the 20th century when close parallelism was noted between its actions and the manifestations of certain allergic reactions. It was implicated as a mediator of hypersensitivity Fig. 11.1: Synthesis and degradation of histamine phenomena and tissue injury reactions. It is now MAO-Monoamine oxidase known to play important physiological roles. Histamine is present mostly within storage by Asch and Schild (1966) into H1 and H2 : those granules of mast cells. Tissues rich in histamine blocked by then available antihistamines were are skin, gastric and intestinal mucosa, lungs, liver labelled H1. Sir James Black (1972) developed and placenta. Nonmast cell histamine occurs in the first H2 blocker burimamide and confirmed brain, epidermis, gastric mucosa and growing this classification. A third H3 receptor, which regions. Turnover of mast cell histamine is slow, serves primarily as an autoreceptor controlling while that of nonmast cell histamine is fast. histamine release from neurones in brain was Histamine is also present in blood, most body identified in 1983. Though some selective H3 secretions, venoms and pathological fluids. agonists and antagonists have been produced, none has found any clinical application. Features of Synthesis, storage and destruction these 3 types of histaminergic receptor are Histamine is β imidazolylethylamine. It is compared in Table 11.1. synthesized locally from the amino acid histidine Molecular cloning has revealed yet another (H4) receptor and degraded rapidly by oxidation and methylation in 2001. -

Empty Nose Syndrome
EMPTY NOSE SYNDROME 1 Empty Nose Syndrome 1.1 Definition The descriptive term “Empty nose syndrome” (ENS) was first coined by Kern and Stenkvist in 1994, who described the condition with substantial loss of tissue inside the nose in the region of the inferior and middle turbinates as “empty nose” (Scheithauer, 2010). “Empty nose” patients suffer from chronic nasal crusting, dryness, and intermittent bleeding. The most distinctive symptom of ENS is “paradoxical obstruction”- in that the patient suffers from difficulty breathing despite a widely patent nasal airway (Sozansky & Houser, 2014). ENS patients describe their feeling of nasal obstruction as constant and continuous: feeling of suffocation, inability or significant difficulty to breathe through their nose, feeling that their nose is too open, sensation of excessive airflow, shortness of breath, difficulty to properly inflate the lungs, lack of nasal resistance, and/or undifferentiated breathing difficulties (Houser, 2006; Houser, 2007; Chhabra & Houser, 2009; Scheithauer, 2010; Coste et al, 2012). 1.2 Primary symptoms ENS patients may experience various debilitating and devastating symptoms, in varying degrees, that can occur after excessive resection of functioning turbinate tissue from the nasal cavity (Moore & Kern, 2001). While the primary symptom is nasal airway obstruction, other symptoms depend upon the type of surgery, extent of functional nasal tissue removal, nature of the bacterial organisms growing inside the nasal cavity, and other unidentified factors. The set and level of severity of symptoms depends upon each individual case. ENS-related symptoms can be divided into three major categories and may include: Respiratory: paradoxical obstruction (feeling of suffocation, inability or significant difficulty to breathe through the nose, feeling that the nose is too open, sensation of excessive airflow, lack of nasal resistance, shortness of breath (dyspnea), difficulty to properly inflate the lungs, difficulty drawing a full breath, weakened airflow, and undifferentiated breathing difficulties) 1. -
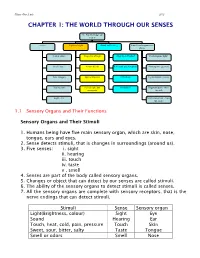
Chapter 1: the World Through Our Senses
Science Form 2 note 2012 CHAPTER 1: THE WORLD THROUGH OUR SENSES The World through our senses senses Light and sight Sound and hearing Stimuli and responses in plants Touch (skin) Properties of light Properties of sound Phototropism (light) Smell (nose) Vision defects Reflection and absorption Geotropism (gravity) Taste (tongue) Optical illusions limitations Hydrotropism (water) Hearing (ear) Stereoscopic and stereophonic Thigmotropism (move monocular toward) Sight (eye) Nastic movement (move run away) 1.1 Sensory Organs and Their Functions Sensory Organs and Their Stimuli 1. Humans being have five main sensory organ, which are skin, nose, tongue, ears and eyes. 2. Sense detects stimuli, that is changes in surroundings (around us). 3. Five senses: i. sight ii. hearing iii. touch iv. taste v . smell 4. Senses are part of the body called sensory organs. 5. Changes or object that can detect by our senses are called stimuli. 6. The ability of the sensory organs to detect stimuli is called senses. 7. All the sensory organs are complete with sensory receptors, that is the nerve endings that can detect stimuli. Stimuli Sense Sensory organ Light(Brightness, colour) Sight Eye Sound Hearing Ear Touch, heat, cold, pain, pressure Touch Skin Sweet, sour, bitter, salty Taste Tongue Smell or odors Smell Nose Science Form 2 note 2012 Laman web. http://freda.auyeung.net/5senses/see.htm http://freda.auyeung.net/5senses/touch.htm http://freda.auyeung.net/5senses/hear.htm http://freda.auyeung.net/5senses/taste.htm http://freda.auyeung.net/5senses/smell.htm 1.2 The Pathway from Stimulus to Response PMR 05 Stimulus Sensory organs Nerves Brain Nerve Response Figure 1.2 The summary of the pathway from stimulus to response 1. -

Toxicological Profile for Acetone Draft for Public Comment
ACETONE 1 Toxicological Profile for Acetone Draft for Public Comment July 2021 ***DRAFT FOR PUBLIC COMMENT*** ACETONE ii DISCLAIMER Use of trade names is for identification only and does not imply endorsement by the Agency for Toxic Substances and Disease Registry, the Public Health Service, or the U.S. Department of Health and Human Services. This information is distributed solely for the purpose of pre dissemination public comment under applicable information quality guidelines. It has not been formally disseminated by the Agency for Toxic Substances and Disease Registry. It does not represent and should not be construed to represent any agency determination or policy. ***DRAFT FOR PUBLIC COMMENT*** ACETONE iii FOREWORD This toxicological profile is prepared in accordance with guidelines developed by the Agency for Toxic Substances and Disease Registry (ATSDR) and the Environmental Protection Agency (EPA). The original guidelines were published in the Federal Register on April 17, 1987. Each profile will be revised and republished as necessary. The ATSDR toxicological profile succinctly characterizes the toxicologic and adverse health effects information for these toxic substances described therein. Each peer-reviewed profile identifies and reviews the key literature that describes a substance's toxicologic properties. Other pertinent literature is also presented, but is described in less detail than the key studies. The profile is not intended to be an exhaustive document; however, more comprehensive sources of specialty information are referenced. The focus of the profiles is on health and toxicologic information; therefore, each toxicological profile begins with a relevance to public health discussion which would allow a public health professional to make a real-time determination of whether the presence of a particular substance in the environment poses a potential threat to human health. -
Investigating the Practical Use of Computational Fluid Dynamics Simulation of Airflow in the Nasal Cavity and Paranasal Sinuses NOSE Version 1.0 2018-08-30
NOSE Pilot Study Investigating the Practical Use of Computational Fluid Dynamics Simulation of Airflow in the NNOSEasal Cavity and Paranasal Sinuses NOSE Pilot Study Investigating the Practical Use of Computational Fluid Dynamics Simulation of Airflow in the Nasal Cavity and Paranasal Sinuses NOSE Version 1.0 2018-08-30 NOSE Pilot Study Investigating the Practical Use of Computational Fluid Dynamics Simulation Version 1.0 of Airflow in the Nasal Cavity and Paranasal Sinuses NOSE Pilot Study Investigating the Practical Use of Computational Fluid Dynamics Simulation of Airflow in the Nasal Cavity and Paranasal Sinuses Project Number 1000043433 Project Title NOSE Pilot Study Document Reference Investigating the Practical Use of Computational Date 2018-08-30 Title Fluid Dynamics Simulation of Airflow in the Nasal Cavity and Paranasal Sinuses Document Name NOSE_PS_FINAL_v1 Version Draft draft final Restrictions public internal restricted : Distribution Steirische Forschungsförderung Authors Koch Walter, Koch Gerda, Vitiello Massimo, Ortiz Ramiro, Stockklauser Jutta, Benda Odo Abstract The NOSE Pilot study evaluated the technical and scientific environment required for establishing a service portfolio that includes CFD simulation and 3D visualization services for ENT specialists. For this purpose the state-of-the-art of these technologies and their use for upper airways diagnostics were analysed. Keywords Rhinology, Computational Fluid Dynamics, 3D Visualization, Clinical Pathways, Service Center, Knowledge Base Document Revisions Version Date Author(s) Description of Change 1.0 2018-08-30 Koch Walter, Koch Final Version Gerda, Vitiello Massimo, Ortiz NOSERamiro, Stockklauser Jutta, Benda Odo 2018-08-30 Seite 3 / 82 Copyright © AIT ForschungsgesmbH NOSE_PS_FINAL_v1 NOSE Pilot Study Investigating the Practical Use of Computational Fluid Dynamics Simulation Version 1.0 of Airflow in the Nasal Cavity and Paranasal Sinuses Table of Contents Acknowledgments .................................................................................. -

Current Awareness in Clinical Toxicology Editors: Damian Ballam Msc and Allister Vale MD
Current Awareness in Clinical Toxicology Editors: Damian Ballam MSc and Allister Vale MD January 2017 CONTENTS General Toxicology 11 Metals 38 Management 21 Pesticides 39 Drugs 23 Chemical Warfare 41 Chemical Incidents & 33 Plants 41 Pollution Chemicals 33 Animals 42 CURRENT AWARENESS PAPERS OF THE MONTH 2015 Annual Report of the American Association of Poison Control Centers' National Poison Data System (NPDS): 33rd Annual Report Mowry JB, Spyker DA, Brooks DE, Zimmerman A, Schauben JL. Clin Toxicol 2016; 54: 924-1109. Introduction This is the 33rd Annual Report of the American Association of Poison Control Centers' (AAPCC) National Poison Data System (NPDS). As of 1 January 2015, 55 of the nation's poison centers (PCs) uploaded case data automatically to NPDS. The upload interval was 9.52 [7.40, 13.6] (median [25%, 75%]) minutes, creating a near real-time national exposure and information database and surveillance system. Methods We analyzed the case data tabulating specific indices from NPDS. The methodology was similar to that of previous years. Where changes were introduced, the differences are identified. Poison center cases with medical outcomes of death were evaluated by a team of medical and clinical toxicologist reviewers using an ordinal scale of 1-6 to assess the Relative Contribution to Fatality (RCF) of the exposure. Results In 2015, 2,792,130 closed encounters were logged by NPDS: 2,168,371 human exposures, 55,516 animal exposures, 560,467 information calls, 7657 human confirmed nonexposures, Current Awareness in Clinical Toxicology is produced monthly for the American Academy of Clinical Toxicology by the Birmingham Unit of the UK National Poisons Information Service, with contributions from the Cardiff, Edinburgh, and Newcastle Units.