New Zealand Journal of Botany How
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Plant Charts for Native to the West Booklet
26 Pohutukawa • Oi exposed coastal ecosystem KEY ♥ Nurse plant ■ Main component ✤ rare ✖ toxic to toddlers coastal sites For restoration, in this habitat: ••• plant liberally •• plant generally • plant sparingly Recommended planting sites Back Boggy Escarp- Sharp Steep Valley Broad Gentle Alluvial Dunes Area ment Ridge Slope Bottom Ridge Slope Flat/Tce Medium trees Beilschmiedia tarairi taraire ✤ ■ •• Corynocarpus laevigatus karaka ✖■ •••• Kunzea ericoides kanuka ♥■ •• ••• ••• ••• ••• ••• ••• Metrosideros excelsa pohutukawa ♥■ ••••• • •• •• Small trees, large shrubs Coprosma lucida shining karamu ♥ ■ •• ••• ••• •• •• Coprosma macrocarpa coastal karamu ♥ ■ •• •• •• •••• Coprosma robusta karamu ♥ ■ •••••• Cordyline australis ti kouka, cabbage tree ♥ ■ • •• •• • •• •••• Dodonaea viscosa akeake ■ •••• Entelea arborescens whau ♥ ■ ••••• Geniostoma rupestre hangehange ♥■ •• • •• •• •• •• •• Leptospermum scoparium manuka ♥■ •• •• • ••• ••• ••• ••• ••• ••• Leucopogon fasciculatus mingimingi • •• ••• ••• • •• •• • Macropiper excelsum kawakawa ♥■ •••• •••• ••• Melicope ternata wharangi ■ •••••• Melicytus ramiflorus mahoe • ••• •• • •• ••• Myoporum laetum ngaio ✖ ■ •••••• Olearia furfuracea akepiro • ••• ••• •• •• Pittosporum crassifolium karo ■ •• •••• ••• Pittosporum ellipticum •• •• Pseudopanax lessonii houpara ■ ecosystem one •••••• Rhopalostylis sapida nikau ■ • •• • •• Sophora fulvida west coast kowhai ✖■ •• •• Shrubs and flax-like plants Coprosma crassifolia stiff-stemmed coprosma ♥■ •• ••••• Coprosma repens taupata ♥ ■ •• •••• •• -

Pteridologist 2007
PTERIDOLOGIST 2007 CONTENTS Volume 4 Part 6, 2007 EDITORIAL James Merryweather Instructions to authors NEWS & COMMENT Dr Trevor Walker Chris Page 166 A Chilli Fern? Graham Ackers 168 The Botanical Research Fund 168 Miscellany 169 IDENTIFICATION Male Ferns 2007 James Merryweather 172 TREE-FERN NEWSLETTER No. 13 Hyper-Enthusiastic Rooting of a Dicksonia Andrew Leonard 178 Most Northerly, Outdoor Tree Ferns Alastair C. Wardlaw 178 Dicksonia x lathamii A.R. Busby 179 Tree Ferns at Kells House Garden Martin Rickard 181 FOCUS ON FERNERIES Renovated Palace for Dicksoniaceae Alastair C. Wardlaw 184 The Oldest Fernery? Martin Rickard 185 Benmore Fernery James Merryweather 186 FEATURES Recording Ferns part 3 Chris Page 188 Fern Sticks Yvonne Golding 190 The Stansfield Memorial Medal A.R. Busby 191 Fern Collections in Manchester Museum Barbara Porter 193 What’s Dutch about Dutch Rush? Wim de Winter 195 The Fine Ferns of Flora Græca Graham Ackers 203 CONSERVATION A Case for Ex Situ Conservation? Alastair C. Wardlaw 197 IN THE GARDEN The ‘Acutilobum’ Saga Robert Sykes 199 BOOK REVIEWS Encyclopedia of Garden Ferns by Sue Olsen Graham Ackers 170 Fern Books Before 1900 by Hall & Rickard Clive Jermy 172 Britsh Ferns DVD by James Merryweather Graham Ackers 187 COVER PICTURE: The ancestor common to all British male ferns, the mountain male fern Dryopteris oreades, growing on a ledge high on the south wall of Bealach na Ba (the pass of the cattle) Unless stated otherwise, between Kishorn and Applecross in photographs were supplied the Scottish Highlands - page 172. by the authors of the articles PHOTO: JAMES MERRYWEATHER in which they appear. -

Pteridologist 2009
PTERIDOLOGIST 2009 Contents: Volume 5 Part 2, 2009 The First Pteridologist Alec Greening 66 Back from the dead in Corrie Fee Heather McHaffie 67 Fern fads, fashions and other factors Alec Greening 68 A Stumpery on Vashon Island near Seattle Pat Reihl 71 Strange Revisions to The Junior Oxford English Dictionary Alistair Urquhart 73 Mauchline Fern Ware Jennifer Ide 74 More Ferns In Unusual Places Bryan Smith 78 The Ptéridophytes of Réunion Edmond Grangaud 79 Croziers - a photographic study. Linda Greening 84 A fern by any other name John Edgington 85 Tree-Fern Newsletter No. 15 Edited by Alastair C. Wardlaw 88 Editorial: TFNL then and now Alastair C. Wardlaw 88 Courtyard Haven for Tree Ferns Alastair C. Wardlaw 88 Bulbils on Tree Ferns: II Martin Rickard 90 Gough-Island Tree Fern Comes to Scotland Jamie Taggart 92 Growing ferns in a challenging climate Tim Pyner 95 Maraudering caterpillars. Yvonne Golding 104 New fern introductions from Fibrex Nurseries Angela Tandy 105 Ferns which live with ants! Yvonne Golding 108 The British Fern Gazette 1909 – 2009 Martin Rickard 110 A Siberian Summer Chris Page 111 Monitoring photographs of Woodsia ilvenis Heather McHaffie 115 Notes on Altaian ferns Irina Gureyeva 116 Ferns from the Galapagos Islands. Graham Ackers 118 Did you know? (Extracts from the first Pteridologist) Jimmy Dyce 121 The First Russian Pteridological Conference Chris Page 122 Tectaria Mystery Solved Pat Acock 124 Chatsworth - a surprising fern link with the past Bruce Brown 125 Fern Postage Stamps from the Faroe Islands Graham Ackers 127 Carrying out trials in your garden Yvonne Golding 128 A national collection of Asplenium scolopendrium Tim Brock 130 Asplenium scolopendrium ‘Drummondiae’ Tim Brock 132 Fern Recording – A Personal Scottish Experience Frank McGavigan 133 Book Notes Martin Rickard 136 Gay Horsetails Wim de Winter 137 Ferning in snow Martin Rickard 139 Fern Enthusiasts do the strangest things. -
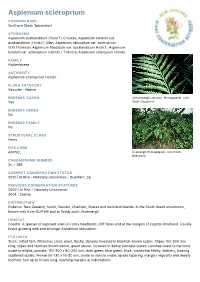
Asplenium Scleroprium
Asplenium scleroprium COMMON NAME Southern Shore Spleenwort SYNONYMS Asplenium aucklandicum (Hook.f.) Crookes; Asplenium lucidum var. aucklandicum (Hook.f.) Allan; Asplenium obtusatum var. scleroprium G.M.Thomson; Asplenium flaccidum var. aucklandicum Hook.f.; Asplenium lucidum var. scleroprium (Hombr.) T.Moore; Asplenium scleropium Hombr. FAMILY Aspleniaceae AUTHORITY Asplenium scleroprium Hombr. FLORA CATEGORY Vascular – Native ENDEMIC TAXON At Invercargill (January). Photographer: John Yes Smith-Dodsworth ENDEMIC GENUS No ENDEMIC FAMILY No STRUCTURAL CLASS Ferns NVS CODE ASPSCL Invercargill. Photographer: John Smith- Dodsworth CHROMOSOME NUMBER 2n = 288 CURRENT CONSERVATION STATUS 2012 | At Risk – Naturally Uncommon | Qualifiers: Sp PREVIOUS CONSERVATION STATUSES 2009 | At Risk – Naturally Uncommon 2004 | Sparse DISTRIBUTION Endemic. New Zealand, South, Stewart, Chatham, Snares and Auckland Islands. In the South Island uncommon, known only from Bluff Hill and at Sandy point, Invercargill. HABITAT Coastal. A species of exposed sites on rocky headlands, cliff faces and at the margins of coastal shrubland. Usually found growing with and amongst Asplenium obtusatum. FEATURES Stout, tufted fern. Rhizomes stout, erect, fleshy, densely invested in blackish-brown scales. Stipes 150-500 mm long, stipes and rachises brown below, green above. Covered in dense subulate scales. Laminae ovate to narrowly ovate or elliptic, pinnate, 150-500 x 80-200 mm, dark green, blue green, thick, somewhat fleshy, leathery, bearing scattered scales. Pinnae 50-130 x 10-20 mm, ovate to narrow ovate, apices tapering, margins regularly and deeply toothed. Sori up to 10 mm long, reaching margins at indentations SIMILAR TAXA Most likely to be confused with A. obtusatum with which it frequently grows and sometimes hybridises with. -

A Planter's Handbook for Northland Natives
A planter’s handbook for Northland natives Including special plants for wetlands, coast and bird food Tiakina nga manu, ka ora te ngahere. Ka ora te ngahere, ka ora nga manu. Look after the birds and the forest flourishes. If the forest flourishes, the birds flourish. Photo courtesy of ????? ACKNOWLEDGEMENTS All photos by Lisa Forester, Katrina Hansen, Jacki Byrd, Brian Chudleigh, Nan Pullman, Malcolm Pullman and Tawapou Coastal Natives. All images copyright of Northland Regional Council unless specified. First published 1999. Updated and reprinted 2020. ISBN: 978-0-909006-65-5. Choosing the right plants Are you deciding on what native Northland plants to use on your land? Whether you’re deciding on plants for landscaping or restoration, this handbook will help. Getting started Photo courtesy of Brian Chudleigh Read on to find out the size and growth rate of plants and which natives attract wildlife. While not listing every plant native to Northland, this book contains a wide range that may be available in local nurseries. Charts on each page show whether a plant provides food for birds, what its final height may be and how quickly it grows. The book also includes plants that will handle harsh coastal environments, windy and/or dry Although primarily a fruit locations and frosts, as well as those plants eater the kūkupa will that tolerate shade or a wetter habitat. This sometimes eat the flowers information will help you choose plants that and new shoots of the kōwhai, Sophora microphylla will benefit you, the local wildlife, and the and some other trees, when environment. -

Ecology of Hard Beech (Nothofagus Truncata) in Southern Outlier Stands
A. F. MARK and W. G. LEE 97 Botany Department, University of Otago, P.O. Box 56, Dunedin, New Zealand. Botany Division, D.S.I.R., Private Bag, Dunedin, New Zealand. ECOLOGY OF HARD BEECH (NOTHOFAGUS TRUNCATA) IN SOUTHERN OUTLIER STANDS IN THE HAAST ECOLOGICAL DISTRICT, SOUTH WESTLAND, NEW ZEALAND Summary: Vegetation and habitat descriptions are given for sites that span the very limited environmental range of southern outlier stands of hard beech (Nothofagus truncata). These are on well-drained, north to northwest aspect slopes at 44 oS in South Westland, 260km south of the species' previously assumed southern limit. Size class distributions and diameter growth rates of hard beech stems indicate that it is competing effectively with podocarp and broadleaved species, including the two other beeches present. Of the three local species (mountain beech - N. solandri var. cliffortioides and silver beech - N. menziesii), only hard beech showed a significant relationship between stem diameter and age, though diameter growth rates were generally similar among the three species. The erratic distribution of the three local beech species in the Haast and adjacent Paringa Ecological Districts is discussed in relation to possible glacial refugia. The scientific and conservation values of the outlier stands are emphasised. Keywords: Nothofagus truncata; Nothofagus biogeography; hard beech forest; tree age-size relations; glacial refugia; South Westland, New Zealand. Introduction June (1977) briefly described the forest The unexpected recent discovery of hard beech communities associated with hard beech as ranging (Nothofagus truncata) at five lowland localities near 'from tall forest where Dacrydium cupressinum', the Arawata and Waiatoto Rivers in the Haast Metrosideros umbellata, Nothofagus menziesii, N. -

Key Ecological Sites of Hamilton City Volume I
Key Ecological Sites of Hamilton City Volume I CBER Contract Report 121 Client report prepared for Hamilton City Council by Toni S. Cornes, Rachel E. Thomson, Bruce D. Clarkson Centre for Biodiversity and Ecology Research Department of Biological Sciences Faculty of Science and Engineering The University of Waikato Private Bag 3105 Hamilton, New Zealand May 31st 2012 Email: [email protected] i ii Contents Executive Summary ........................................................................................................................ 1 Report Context and Overview...................................................................................................... 2 Overview .......................................................................................................................................... 2 Hamilton City Boundaries ................................................................................................................ 3 Ecology of Hamilton ......................................................................................................................... 4 Climate ......................................................................................................................................... 4 Geology ........................................................................................................................................ 4 Landforms and Vegetation Types ................................................................................................ 4 Fauna of Hamilton -

Appendix 6. Assessment of Ecological Effects
Appendix 6. Assessment of Ecological Effects 84 ASSESSMENT OF ECOLOGICAL EFFECTS FOR A PROPOSED ZIP LINE TOUR NEAR HUKA FALLS R5077 ASSESSMENT OF ECOLOGICAL EFFECTS FOR A PROPOSED ZIP LINE TOUR NEAR HUKA FALLS View of kōhūhū-kānuka-whauwhaupaku forest within Huka Falls Scenic Reserve from Ferguson’s Gully track. 26 June 2019. Contract Report No. 5077 August 2019 Project Team: Angela Simpson - Report author William Shaw - Project management Prepared for: Sky Play Adventures Taupō 99 SALA STREET, WHAKAREWAREWA, 3010, P.O. BOX 7137, TE NGAE, ROTORUA 3042 Ph 07-343-9017; Fax 07-343-9018, email [email protected], www.wildlands.co.nz CONTENTS 1. INTRODUCTION 1 2. METHODS 1 2.1 Overview 1 2.2 Review of existing information 1 2.3 Site visit 2 2.4 Assessment of potential effects 2 3. OVERVIEW OF PROPOSED ZIP LINE OPERATION 2 3.1 Overview 2 3.2 Footprint of the proposed operation 3 3.3 Construction methods 3 4. ECOLOGICAL AND STATUTORY CONTEXT 6 4.1 Overview 6 4.2 Atiamuri Ecological District 6 4.3 Huka Falls Scenic Reserve 8 4.4 Huka Falls (true left side) 9 4.5 Upper Waikato River 9 4.6 Ecological restoration in the area 9 4.7 District plan provisions 11 5. VEGETATION AND HABITATS AT THE SITE 11 6. VEGETATION AND HABITATS AT SPECIFIC LOCATIONS 13 6.1 Overview 13 6.2 Tower 1 13 6.3 Platform 2 14 6.4 Platform 3 15 6.5 Track from Platform 3 to Platform 4 15 6.6 Platform 4 17 6.7 Platform 5 17 6.8 Platform 6 17 6.9 Track from Platform 6 to Tower 7 20 6.10 Tower 7 23 6.11 Tower 8 23 7. -

Aspleniaceae on the South Coast of New South
Cunninghamia Date of Publication: October 2019 A journal of plant ecology for eastern Australia ISSN 0727- 9620 (print) • ISSN 2200 - 405X (Online) The coastal ferns Asplenium decurrens and Asplenium difforme (Aspleniaceae) on the south coast of New South Wales. Kevin Mills 12 Hyam Place, Jamberoo, NSW 2533 AUSTRALIA. [email protected] Abstract: The occurrence of the coastal ferns Asplenium decurrens and Asplenium difforme (family Aspleniaceae) on the south coast of New South Wales are assessed and discussed. The Illawarra coast was the zone of overlap for these two coastal fern species and they reached their geographical limits in the Kiama area, with Asplenium decurrens reaching its northern limit of distribution and Asplenium difforme reaching its southern limit. All substantiated records of these ferns are documented and assessed. Both species require conservation consideration on the NSW south coast. For Asplenium decurrens the evidence suggests that New South Wales should follow Victoria and list this species as threatened under the Biodiversity Conservation Act 2016 (NSW). For Asplenium difforme local conservation action is needed at its most southern limit at Kiama. Cunninghamia (2019) 19: 107–111 doi: 10.7751/cunninghamia.2019.19.009 Cunninghamia: a journal of plant ecology for eastern Australia © 2019 Royal Botanic Gardens and Domain Trust www.rbgsyd.nsw.gov.au/science/Scientific_publications/cunninghamia 108 Cunninghamia 19: 2019 Mills, South Coast ferns Asplenium decurrens and Asplenium difforme Introduction as a co-author, re-instated the original name Asplenium decurrens Willd.; Brownsey & Perrie (2016) set out the The coastal ferns Asplenium decurrens (syn. Asplenium reasoning for re-instating the original name. -

Flora and Vegetation of the Cavalli Islands (Except Motukawanui), Northern New Zealand
TANK 25, 1979 FLORA AND VEGETATION OF THE CAVALLI ISLANDS (EXCEPT MOTUKAWANUI), NORTHERN NEW ZEALAND by A.E. Wright Department of Botany, University of Auckland, Private Bag, Auckland SUMMARY A combined flora of 242 taxa of higher plants is presented for 28 vegetated islands (excepting Motukawanui, the largest island) in the Cavalli Island Group. The islands range from small wind- and sea-swept rock stacks to 38 hectare Motukawanui (Step Island) which is farmed. Analysis of the combined flora indicates a high proportion (46%) of adventive plants, due largely to unstable soil conditions and interference with the vegetation by man. A large number of taxa (30%) are confined to one island only, giving evidence of the wide variety of habitats and vegetation com• munities to be found. A brief vegetation description and individual checklists of plants are given for each island and group of rock stacks visited. Unusual plant com• munities and those of largely indigenous composition are treated in more detail. The rare native plant Hibiscus trionum is noted from three islands, on one of which many thousands of plants occur. Finally, a number of general observations on the vegetation of the islands are discussed, and priorities for management and reservation on botanical grounds are given. INTRODUCTION The Cavalli Island Group (latitude 35° 00'S, longitude 173° 57'E) lies between two and seven kilometres off the Northland coast and about 16 kilometres east of the entrance to Whangaroa Harbour (Fig. 1). Some thirty vegetated islands and groups of rocks lie scattered around Motukawanui, the largest island, and are geographically divisible into four main groups. -

Plant Species List of Erua Wetland, Riparian Forest and Scarp, Part Of
Plant species List of Erua wetland, riparian forest and scarp, part of Erua Conservation Area, 60097 File number: DOCDM−392501 This list incorporates all vascular plants found west of SH 4 and the top of the Erua fault scarp, east of Erua Road including the wetland, alluvial terraces, fault scarp and talus slope This list is incorporates several lists of indigenous vascular plants over many years. • Ecroyd and Bergin (1981). Ecroyd and Bergin cover the area from N111/885722 on the Erua road in the north to N111/870705 at the southern end of the wetland, including the eastern side of the Waimarino River. • Druce and Ogle (1982) cover the area between the Erua road on the west and Waimarino river on the east, from Erua road (NZMS 1, N111/881723) in the north to N121/865696 in the south, and include species in the large wetland, as well as the native forest near the road. • Data from C.E. Ecroyd and B.N Spring-Rice 1992, 1994 and 1995 plot records from block A. Block A is the northern stand of Pinus ponderosa, east of the Waimarino River to the “White horse” forestry access road and S.H.4. • Additions to block A, next to the Waimarino River and adventives from December 1998 by N.J.D. Singers and Cate P. Ryanφ • Additions by C.C. Ogle, and N.J.D. Singers 11th January 2002 φ • Additions by C.C. Ogle, N.J.D. Singers and R.B.Whyman February 3rd 2003 # • Additions by C.C.Ogle, P.de Lange and Nicholas Singers 14th and 16th February 2005 ¥ • Additions by Nicholas Singers, 9th January 2009 ∫. -

Issn 0811-5311 Date -June, 2000
'? ASSOCIATION of ISSN 0811-5311 DATE -JUNE, 2000 LEADER Peter Hind, 41 Miller Street, Mount Druitt. N. S. W. 2770 SECRETARY: Vacant TREASURER: Joan Moore, 2 Gannet Street, Gladesville. N. S.W. 2 1 11 NEWSLETTER EDITOR: Mike Healy, 272 HumMiay St. Nth., Ballarat. Vic. 3350 E-mail address: [email protected]> (N.B. It is n mhealy) SPORE BANK: Barry Wlute, 24 Ruby Street, West Essendon. Vic. 3040 .................................................................................. ASGAP FERN STUDY GROUP FINANCIAL REPORT- Reprinted due to format errors in March Newsletter Statement of Receipts and Payments for the I999 Calendar Year RECEIPTS 1999 Previous Year Members' Subscriptions $515 $484 (includes some in advance) Donations 150 75 S.G.A.P. Regions I35 Members I5 Raffles-Sydney Meeting 54 6 5 Sale of Book. 45 Interest received -2.54 5 Total Receipts 766.54 626 PAYMENTS Newsletter Expenses: Paper and Printing Postage Stationery Postage - Correspondence Bank Charges, F.I.D. and Money orders Donation to Burrendong Arboretum Photos for Fern Book Total Payments SURPLUS [DEFICIT) FOR YEAR 3 0.28 (748) SUMMARY Cash at bank at beginning of year Surplus for year Cash at bank at end of year APOLOGY - The Editor apologises to Treasurer, Joan Moore, for any embarrassment or concern she may have experienced as a result of the formatting errors in the Annual Financial Report. I wish to reassure Joan that readers of this Newsletter are discerning enough to identify that this was not an error reflecting on her competence. Clearly it was error in reproduction and oversight in proof reading. Unfortunately, like most people, I do make mistakes, on occasion.