Open Thesis.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Characterization of L-Serine Deaminases, Sdaa (PA2448) and Sdab 2 (PA5379), and Their Potential Role in Pseudomonas Aeruginosa 3 Pathogenesis 4 5 Sixto M
bioRxiv preprint doi: https://doi.org/10.1101/394957; this version posted August 20, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 1 Characterization of L-serine deaminases, SdaA (PA2448) and SdaB 2 (PA5379), and their potential role in Pseudomonas aeruginosa 3 pathogenesis 4 5 Sixto M. Leal1,6, Elaine Newman2 and Kalai Mathee1,3,4,5 * 6 7 Author affiliations: 8 9 1Department of Biological Sciences, College of Arts Sciences and 10 Education, Florida International University, Miami, United States of 11 America 12 2Department of Biological Sciences, Concordia University, Montreal, 13 Canada 14 3Department of Molecular Microbiology and Infectious Diseases, Herbert 15 Wertheim College of Medicine, Florida International University, Miami, 16 United States of America 17 4Biomolecular Sciences Institute, Florida International University, Miami, 18 United States of America 19 20 Present address: 21 22 5Department of Human and Molecular Genetics, Herbert Wertheim 23 College of Medicine, Florida International University, Miami, United States 24 of America 25 6Case Western Reserve University, United States of America 26 27 28 *Correspondance: Kalai Mathee, MS, PhD, 29 [email protected] 30 31 Telephone : 1-305-348-0628 32 33 Keywords: Serine Catabolism, Central Metabolism, TCA Cycle, Pyruvate, 34 Leucine Responsive Regulatory Protein (LRP), One Carbon Metabolism 35 Running title: P. aeruginosa L-serine deaminases 36 Subject category: Pathogenicity and Virulence/Host Response 37 1 bioRxiv preprint doi: https://doi.org/10.1101/394957; this version posted August 20, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. -

Reduction by the Methylreductase System in Methanobacterium Bryantii WILLIAM B
JOURNAL OF BACTERIOLOGY, Jan. 1987, p. 87-92 Vol. 169, No. 1 0021-9193/87/010087-06$02.00/0 Copyright © 1987, American Society for Microbiology Inhibition by Corrins of the ATP-Dependent Activation and CO2 Reduction by the Methylreductase System in Methanobacterium bryantii WILLIAM B. WHITMAN'* AND RALPH S. WOLFE2 Department of Microbiology, University of Georgia, Athens, Georgia 30602,1 and Department of Microbiology, University ofIllinois, Urbana, Illinois 618012 Received 1 August 1986/Accepted 28 September 1986 Corrins inhibited the ATP-dependent activation of the methylreductase system and the methyl coenzyme M-dependent reduction of CO2 in extracts of Methanobacterium bryantii resolved from low-molecular-weight factors. The concentrations of cobinamides and cobamides required for one-half of maximal inhibition of the ATP-depen4ent activation were between 1 and 5 ,M. Cobinamides were more inhibitory at lower concentra- tiops than cobamides. Deoxyadenosylcobalamin was not inhibitory at concentrations up to 25 ,uM. The inhibition of CO2 reduction was competitive with respect to CO2. The concentration of methylcobalamin required for one-half of maximal inhibition was 5 ,M. Other cobamideg inhibited at similar concentrations, but diaquacobinami4e inhibited at lower concentrations. With respect to their affinities and specificities for corrins, inhibition of both the ATP-dependent activation'and CO2 reduction closely resembled the corrin- dependent activation of the methylreductase described in similar extracts (W. B. Whitman and R. S. Wolfe, J. Bacteriol. 164:165-172, 1985). However, whether the multiple effects of corrins are due to action at a single site is unknown. The effect of corrins (cobamides and cobinamides) on in CO2 reduction. -

Subject Index
Cambridge University Press 0521462924 - Amino Acids and Peptides G. C. Barrett and D. T. Elmore Index More information Subject index acetamidomalonate synthesis 123–4 in fossil dating 15 S-adenosyl--methionine 11, 12, 174, 181 in geological samples 15 alanine, N-acetyl 9 in Nature 1 structure 4 IR spectrometry 36 alkaloids, biosynthesis from amino acids 16–17 isolation from proteins 121 alloisoleucine 6 mass spectra 61 allosteric change 178 metabolism, products of 187 allothreonine 6 metal-binding properties 34 Alzheimer’s disease 14 NMR 41 amidation at peptide C-terminus 57, 94, 181 physicochemical properties 32 amides, cis–trans isomerism 20 protein 3 N-acylation 72 PTC derivatives 87 N-alkylation 72 quaternary ammonium salts 50 hydrolysis 57 reactions of amino group 49, 51 O-trimethylsilylation 72 reactions of carboxy group 49, 53 reduction to -aminoalkanols 72 racemisation 56 amidocarbonylation in amino acid synthesis 125 routine spectrometry 35 amino acids, abbreviated names 7 Schöllkopf synthesis 127–8 acid–base properties 32 Schiff base formation 49 antimetabolites 200 sequence determination 97 et seq. as food additives 14 following selective chemical degradation 107 as neurotransmitters 17 following selective enzymic degradation 109 asymmetric synthesis 127 general strategy for 92 - 17–18 identification of C-terminus 106–7 biosynthesis 121 identification of N-terminus 94, 97 biosynthesis from, of creatinine 183 by solid-phase methodology 100 of nitric oxide 186 by stepwise chemical degradation 97 of porphyrins 185 by use of dipeptidyl -
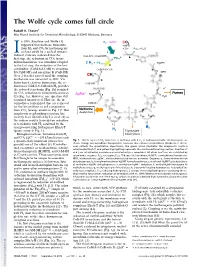
The Wolfe Cycle Comes Full Circle
The Wolfe cycle comes full circle Rudolf K. Thauer1 Max Planck Institute for Terrestrial Microbiology, D-35043 Marburg, Germany n 1988, Rouvière and Wolfe (1) H - ΔμNa+ 2 CO2 suggested that methane formation + MFR from H and CO by methanogenic + 2H+ *Fd + H O I 2 2 ox 2 archaea could be a cyclical process. j O = Indirect evidence indicated that the CoB-SH + CoM-SH fi *Fd 2- a rst step, the reduction of CO2 to for- red R mylmethanofuran, was somehow coupled + * H MPT 2 H2 Fdox 4 to the last step, the reduction of the het- h erodisulfide (CoM-S-S-CoB) to coenzyme CoM-S-S-CoB b MFR M (CoM-SH) and coenzyme B (CoB-SH). H Over 2 decades passed until the coupling C 4 10 mechanism was unraveled in 2011: Via g flavin-based electron bifurcation, the re- CoB-SH duction of CoM-S-S-CoB with H provides 2 H+ the reduced ferredoxin (Fig. 1h) required c + Purines for CO2 reduction to formylmethanofuran ΔμNa + H MPT 4 f H O (2) (Fig. 1a). However, one question still 2 remained unanswered: How are the in- termediates replenished that are removed CoM-SH for the biosynthesis of cell components H Methionine d from CO2 (orange arrows in Fig. 1)? This Acetyl-CoA e anaplerotic (replenishing) reaction has F420 F420H2 recently been identified by Lie et al. (3) as F420 F420H2 the sodium motive force-driven reduction H i of ferredoxin with H2 catalyzed by the i energy-converting hydrogenase EhaA-T H2 (green arrow in Fig. -

Annotation Guidelines for Experimental Procedures
Annotation Guidelines for Experimental Procedures Developed By Mohammed Alliheedi Robert Mercer Version 1 April 14th, 2018 1- Introduction and background information What is rhetorical move? A rhetorical move can be defined as a text fragment that conveys a distinct communicative goal, in other words, a sentence that implies an author’s specific purpose to readers. What are the types of rhetorical moves? There are several types of rhetorical moves. However, we are interested in 4 rhetorical moves that are common in the method section of a scientific article that follows the Introduction Methods Results and Discussion (IMRaD) structure. 1- Description of a method: It is concerned with a sentence(s) that describes experimental events (e.g., “Beads with bound proteins were washed six times (for 10 min under rotation at 4°C) with pulldown buffer and proteins harvested in SDS-sample buffer, separated by SDS-PAGE, and analyzed by autoradiography.” (Ester & Uetz, 2008)). 2- Appeal to authority: It is concerned with a sentence(s) that discusses the use of standard methods, protocols, and procedures. There are two types of this move: - A reference to a well-established “standard” method (e.g., the use of a method like “PCR” or “electrophoresis”). - A reference to a method that was previously described in the literature (e.g., “Protein was determined using fluorescamine assay [41].” (Larsen, Frandesn and Treiman, 2001)). 3- Source of materials: It is concerned with a sentence(s) that lists the source of biological materials that are used in the experiment (e.g., “All microalgal strains used in this study are available at the Elizabeth Aidar Microalgae Culture Collection, Department of Marine Biology, Federal Fluminense University, Brazil.” (Larsen, Frandesn and Treiman, 2001)). -

Yeast Genome Gazetteer P35-65
gazetteer Metabolism 35 tRNA modification mitochondrial transport amino-acid metabolism other tRNA-transcription activities vesicular transport (Golgi network, etc.) nitrogen and sulphur metabolism mRNA synthesis peroxisomal transport nucleotide metabolism mRNA processing (splicing) vacuolar transport phosphate metabolism mRNA processing (5’-end, 3’-end processing extracellular transport carbohydrate metabolism and mRNA degradation) cellular import lipid, fatty-acid and sterol metabolism other mRNA-transcription activities other intracellular-transport activities biosynthesis of vitamins, cofactors and RNA transport prosthetic groups other transcription activities Cellular organization and biogenesis 54 ionic homeostasis organization and biogenesis of cell wall and Protein synthesis 48 plasma membrane Energy 40 ribosomal proteins organization and biogenesis of glycolysis translation (initiation,elongation and cytoskeleton gluconeogenesis termination) organization and biogenesis of endoplasmic pentose-phosphate pathway translational control reticulum and Golgi tricarboxylic-acid pathway tRNA synthetases organization and biogenesis of chromosome respiration other protein-synthesis activities structure fermentation mitochondrial organization and biogenesis metabolism of energy reserves (glycogen Protein destination 49 peroxisomal organization and biogenesis and trehalose) protein folding and stabilization endosomal organization and biogenesis other energy-generation activities protein targeting, sorting and translocation vacuolar and lysosomal -

Untargetted Metabolomic Exploration of the Mycobacterium Tuberculosis Stress Response to Cinnamon Essential Oil
biomolecules Article Untargetted Metabolomic Exploration of the Mycobacterium tuberculosis Stress Response to Cinnamon Essential Oil Elwira Sieniawska 1,* , Rafał Sawicki 2 , Joanna Golus 2 and Milen I. Georgiev 3,4 1 Chair and Department of Pharmacognosy, Medical University of Lublin, Chodzki 1, 20-093 Lublin, Poland 2 Chair and Department of Biochemistry and Biotechnology, Medical University of Lublin, Chodzki 1, 20-093 Lublin, Poland; [email protected] (R.S.); [email protected] (J.G.) 3 Group of Plant Cell Biotechnology and Metabolomics, The Stephan Angeloff Institute of Microbiology, Bulgarian Academy of Sciences, 139 Ruski Blvd., 4000 Plovdiv, Bulgaria; [email protected] 4 Center of Plant Systems Biology and Biotechnology, 4000 Plovdiv, Bulgaria * Correspondence: [email protected] Received: 6 January 2020; Accepted: 24 February 2020; Published: 26 February 2020 Abstract: The antimycobacterial activity of cinnamaldehyde has already been proven for laboratory strains and for clinical isolates. What is more, cinnamaldehyde was shown to threaten the mycobacterial plasma membrane integrity and to activate the stress response system. Following promising applications of metabolomics in drug discovery and development we aimed to explore the mycobacteria response to cinnamaldehyde within cinnamon essential oil treatment by untargeted liquid chromatography–mass spectrometry. The use of predictive metabolite pathway analysis and description of produced lipids enabled the evaluation of the stress symptoms shown by bacteria. This study suggests that bacteria exposed to cinnamaldehyde could reorganize their outer membrane as a physical barrier against stress factors. They probably lowered cell wall permeability and inner membrane fluidity, and possibly redirected carbon flow to store energy in triacylglycerols. Being a reactive compound, cinnamaldehyde may also contribute to disturbances in bacteria redox homeostasis and detoxification mechanisms. -

Isolation and Nucleotide Sequence of the Cdna for Rat Liver Serine
Proc. Natl. Acad. Sci. USA Vol. 85, pp. 5809-5813, August 1988 Biochemistry Isolation and nucleotide sequence of the cDNA for rat liver serine dehydratase mRNA and structures of the 5' and 3' flanking regions of the serine dehydratase gene (threonine dehydratase/hormonal regulation/consensus sequences) HIROFUMI OGAWA*t, DUNCAN A. MILLER*, TRACY DUNN*, YEU SU*, JAMES M. BURCHAMt, CARL PERAINOt, MOTOJI FUJIOKAt, KAY BABCOCK*, AND HENRY C. PITOT*§ *McArdle Laboratory for Cancer Research, The Medical School, University of Wisconsin, Madison, WI 53706; tDepartment of Biochemistry, Toyama Medical and Pharmaceutical University, Faculty of Medicine, Sugitani, Toyama 930-01, Japan; and tDivision of Biological and Medical Research, Argonne National Laboratory, Argonne, IL 60439 Communicated by Van R. Potter, April 15, 1988 (received for review December 29, 1987) ABSTRACT Rat serine dehydratase cDNA clones were determination of the exact size of DNA complementary to isolated from a Agtll cDNA library on the basis of their serine dehydratase mRNA was made by S1 nuclease and reactivity with monospecific immunoglobulin to the purified sequencing of genomic clones of the regions flanking the enzyme. Using the cDNA insert from a clone that encoded the gene. serine dehydratase subunit as a probe, additional clones were isolated from the same library by plaque hybridization. Nucle- otide sequence analysis of the largest clone obtained showed MATERIALS AND METHODS that it has 1444 base pairs with an open reading frame consisting of 1089 base pairs. The deduced amino acid sequence cDNA Cloning. A rat liver cDNA library constructed in contained sequences of several portions of the serine dehydra- Agtll phage (13) was screened for antibody-reactive plaques tase protein, as determined by Edman degradation. -

Spell Checked 12-13 BP Cards 52-113 Layout 1
Provide a detoxification mechanism Deamination is also an oxidative reaction that occurs under aerobic conditions in all tissues but especially the liver and kidneys. During oxidative deamination, an amino acid is converted into the corresponding keto acid (for energy) by the removal of the amine functional group as ammo- nia and the amine functional group is replaced by the ketone group. The ammonia eventually goes into the urea cycle. Oxidative deamination occurs primarily on glutamic acid because glutamic acid was the end product of many transamination reactions. Glutamate dehydrogenase is an enzyme of the oxidoreductase class that catalyzes the oxidative deamination of glutamate. Ammonia is released, and α-ketoglutarate is formed. Glutamate dehydrogenase is unusual in that it can use either NAD or NADP as a coenzyme. The reversible reaction has a major function in both the synthesis and degradation of glutamic acid and, via transaminases, other amino acids as well. *** Important: Both aspartate aminotransferase (AST) and alanine aminotransferase (ALT) are transaminases (aminotransferases). They are not involved in oxidative deamination reactions. In contrast to transamination reactions that transfer amino groups, oxidative deamination results in the liberation of the amino group as free ammonia. 1. Glutaminase deaminates glutamine to glutamate and ammonium ion; asparagin- Notes ase deaminates asparagine to aspartate and ammonium ion. 2. Glutamate is unique in that it is the only amino acid that undergoes rapid oxidative deamination. + 3. Histidine is deaminated by histidase to form ammonium ion (NH 4) and urocan- ate. 4. Serine and threonine are deaminated by serine dehydratase. Serine is converted to pyruvate, and threonine to α-ketobutyrate (which is decarboxylated oxidatively to form propionyl CoA); ammonium ion is released.. -

Letters to Nature
letters to nature Received 7 July; accepted 21 September 1998. 26. Tronrud, D. E. Conjugate-direction minimization: an improved method for the re®nement of macromolecules. Acta Crystallogr. A 48, 912±916 (1992). 1. Dalbey, R. E., Lively, M. O., Bron, S. & van Dijl, J. M. The chemistry and enzymology of the type 1 27. Wolfe, P. B., Wickner, W. & Goodman, J. M. Sequence of the leader peptidase gene of Escherichia coli signal peptidases. Protein Sci. 6, 1129±1138 (1997). and the orientation of leader peptidase in the bacterial envelope. J. Biol. Chem. 258, 12073±12080 2. Kuo, D. W. et al. Escherichia coli leader peptidase: production of an active form lacking a requirement (1983). for detergent and development of peptide substrates. Arch. Biochem. Biophys. 303, 274±280 (1993). 28. Kraulis, P.G. Molscript: a program to produce both detailed and schematic plots of protein structures. 3. Tschantz, W. R. et al. Characterization of a soluble, catalytically active form of Escherichia coli leader J. Appl. Crystallogr. 24, 946±950 (1991). peptidase: requirement of detergent or phospholipid for optimal activity. Biochemistry 34, 3935±3941 29. Nicholls, A., Sharp, K. A. & Honig, B. Protein folding and association: insights from the interfacial and (1995). the thermodynamic properties of hydrocarbons. Proteins Struct. Funct. Genet. 11, 281±296 (1991). 4. Allsop, A. E. et al.inAnti-Infectives, Recent Advances in Chemistry and Structure-Activity Relationships 30. Meritt, E. A. & Bacon, D. J. Raster3D: photorealistic molecular graphics. Methods Enzymol. 277, 505± (eds Bently, P. H. & O'Hanlon, P. J.) 61±72 (R. Soc. Chem., Cambridge, 1997). -

COMPILATION of AMINO ACIDS, DRUGS, METABOLITES and OTHER COMPOUNDS in MASSTRAK AMINO ACID ANALYSIS SOLUTION Paula Hong, Kendon S
COMPILATION OF AMINO ACIDS, DRUGS, METABOLITES AND OTHER COMPOUNDS IN MASSTRAK AMINO ACID ANALYSIS SOLUTION Paula Hong, Kendon S. Graham, Alexandre Paccou, T homas E. Wheat and Diane M. Diehl INTRODUCTION LC conditions Physiological amino acid analysis is commonly performed to LC System: Waters ACQUITY UPLC® System with TUV monitor and study a wide variety of metabolic processes. A wide Column: MassTrak AAA Column 2.1 x 150 mm, 1.7 µm variety of drugs, foods, and metabolic intermediates that may Column Temp: 43 ˚C be present in biological fluids can appear as peaks in amino Flow Rate: 400 µL/min. acid analysis, therefore, it is important to be able to identify Mobile Phase A: MassTrak AAA Eluent A Concentrate, unknown compounds.1,2,3 The reproducibility and robustness of diluted 1:10 the MassTrak Amino Acid Analysis Solution make this method well Mobile Phase B: MassTrak AAA Eluent B suited to such a study as well.4 Weak Needle Wash: 5/95 Acetonitrile/Water Strong Needle Wash: 95/5 Acetonitrile/Water Gradient: MassTrak AAA Standard Gradient (as provided in kit) Detection: UV @ 260 nm Injection Volume: 1 µL EXPERIMENTAL Injection Mode: Partial Loop with Needle Overfill (PLNO) Compound sample preparation A library of compounds was assembled. Each compound was derivatized individually and spiked into the MassTrak™ AAA Solution Standard prior to chromatographic analysis. The elution RESULTS AND DISCUSSION position of each tested compound could be related to known amino acids. A wide variety of antibiotics, pharmaceutical compounds and metabolite by-products are found in biological fluids. The reten- 1. -

Characterization of an L-Serine Dehydratase Activity in Permeabilized Cells of Brevibacterium Linens ATCC 9175 Dominique Hamouy, M
Characterization of an L-serine dehydratase activity in permeabilized cells of Brevibacterium linens ATCC 9175 Dominique Hamouy, M. J. Desmazeaud To cite this version: Dominique Hamouy, M. J. Desmazeaud. Characterization of an L-serine dehydratase activity in permeabilized cells of Brevibacterium linens ATCC 9175. Le Lait, INRA Editions, 1985, 65 (649_650), pp.103-121. hal-00929050 HAL Id: hal-00929050 https://hal.archives-ouvertes.fr/hal-00929050 Submitted on 1 Jan 1985 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Le Lait (1985), 65 (649-650), 103-121 Characterization of an L-serine dehydratase activity in permeabilized cells of Brevibacterium linens ATCC 9175 Dominique HAMOUY and M. J. DESMAZEAUD* SUMMARV Brevibacterium linens ATCC 9175 praduces large quantities of, ammonia and pyruvate [rom L-serine. This reaction occurs via an L-serine dehydratase (EC.4.2.I.13), whose activity is maximal at the end of exponential growth on rich medium and which abruptly decreases at the beginning of the stationary phase. ft is not possible ta extract the soluble form of the enzyme without a total loss of activity. Subcellular localization studies after creating stable proto- plasts with lysozyme have shawn that a part of the activity is bound ta cell membranes.