National Action Plan for Corona Virus Disease (COVID-19) Pakistan
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

FIRMS in AOR of RD PUNJAB Ser Name of Firm Chemical RD
Appendix-A FIRMS IN AOR OF RD PUNJAB Ser Name of Firm Chemical RD 1 M/s A.A Textile Processing Industries, Faisalabad Hydrochloric Acid Punjab 2 M/s A.B Exports (Pvt) Ltd, Faisalabad Hydrochloric Acid Punjab 3 M/s A.M Associates, Lahore Hydrochloric Acid Punjab 4 M/s A.M Knit Wear, Faisalabad Hydrochloric Acid Punjab 5 M/s A.S Chemical, Multan Hydrochloric Acid Punjab 6 M/s A.T Impex, Lahore Hydrochloric Acid Punjab 7 M/s AA Brothers Chemical Traders, Sialkot Hydrochloric Acid Punjab 8 M/s AA Fabrics, Faisalabad Hydrochloric Acid Punjab 9 M/s AA Spinning Mills Ltd, Faisalabad Hydrochloric Acid Punjab 10 M/s Aala Production Industries (Pvt) Ltd, Faisalabad Hydrochloric Acid Punjab 11 M/s Aamir Chemical Store, Multan Hydrochloric Acid Punjab 12 M/s Abbas Chemicals, Lahore Hydrochloric Acid Punjab M/s Abdul Razaq & Sons Tezab and Spray Centre, 13 Hydrochloric Acid Punjab Toba Tek Singh 14 M/s Abubakar Anees Textiles, Faisalabad Hydrochloric Acid Punjab 15 M/s Acro Chemicals, Lahore Toluene & MEK Punjab 16 M/s Agritech Ltd, Lahore Hydrochloric Acid Punjab 17 M/s Ahmad Chemical Traders, Muridke Hydrochloric Acid Punjab 18 M/s Ahmad Chemmicals, Lahore Hydrochloric Acid Punjab 19 M/s Ahmad Industries (Pvt) Ltd, Khanewal Hydrochloric Acid Punjab 20 M/s Ahmed Chemical Traders, Faisalabad Hydrochloric Acid Punjab 21 M/s AHN Steel, Lahore Hydrochloric Acid Punjab 22 M/s Ajmal Industries, Kamoke Hydrochloric Acid Punjab 23 M/s Ajmer Engineering Electric Works, Lahore Hydrochloric Acid Punjab Hydrochloric Acid & Sulphuric 24 M/s Akbari Chemical Company, -

S# BRANCH CODE BRANCH NAME CITY ADDRESS 1 24 Abbottabad
BRANCH S# BRANCH NAME CITY ADDRESS CODE 1 24 Abbottabad Abbottabad Mansera Road Abbottabad 2 312 Sarwar Mall Abbottabad Sarwar Mall, Mansehra Road Abbottabad 3 345 Jinnahabad Abbottabad PMA Link Road, Jinnahabad Abbottabad 4 131 Kamra Attock Cantonment Board Mini Plaza G. T. Road Kamra. 5 197 Attock City Branch Attock Ahmad Plaza Opposite Railway Park Pleader Lane Attock City 6 25 Bahawalpur Bahawalpur 1 - Noor Mahal Road Bahawalpur 7 261 Bahawalpur Cantt Bahawalpur Al-Mohafiz Shopping Complex, Pelican Road, Opposite CMH, Bahawalpur Cantt 8 251 Bhakkar Bhakkar Al-Qaim Plaza, Chisti Chowk, Jhang Road, Bhakkar 9 161 D.G Khan Dera Ghazi Khan Jampur Road Dera Ghazi Khan 10 69 D.I.Khan Dera Ismail Khan Kaif Gulbahar Building A. Q. Khan. Chowk Circular Road D. I. Khan 11 9 Faisalabad Main Faisalabad Mezan Executive Tower 4 Liaqat Road Faisalabad 12 50 Peoples Colony Faisalabad Peoples Colony Faisalabad 13 142 Satyana Road Faisalabad 585-I Block B People's Colony #1 Satayana Road Faisalabad 14 244 Susan Road Faisalabad Plot # 291, East Susan Road, Faisalabad 15 241 Ghari Habibullah Ghari Habibullah Kashmir Road, Ghari Habibullah, Tehsil Balakot, District Mansehra 16 12 G.T. Road Gujranwala Opposite General Bus Stand G.T. Road Gujranwala 17 172 Gujranwala Cantt Gujranwala Kent Plaza Quide-e-Azam Avenue Gujranwala Cantt. 18 123 Kharian Gujrat Raza Building Main G.T. Road Kharian 19 125 Haripur Haripur G. T. Road Shahrah-e-Hazara Haripur 20 344 Hassan abdal Hassan Abdal Near Lari Adda, Hassanabdal, District Attock 21 216 Hattar Hattar -

Zarb-E-Azb and the State of Security in Pakistan
Prof. Dr. Umbreen Javaid1 Zarb-e-Azb and the State of Security in Pakistan Abstract The state of internal security in Pakistan emerged as a challenge to the state-writ due to the societal fragmentation and rise in extremism and terrorism. Incidents of terrorism linked to TTP developed as the major internal security threat in Pakistan. The failure of PML - (N)’s government in bringing the TTP to the dialogue table coupled with a terrifying rise in number of terror attacks on security personal and soft targets led to the hard stance culminating in a comprehensive joint military operation ‘Zarb-e-Azb’ in North Waziristan (FATA) against TTP’s hideouts and their foreign supporters. The paper will focus on the internal security dynamics of Pakistan in post 9/11scanario and the circumstances that led to the massive, large scale military chase in the history of Pakistan [Zarb-e-Azb] to curtail terrorism and to root out extremism. Keywords Internal Security, Operation Zarb-e-Azb, Pakistan, extremism, FATA, terror Introduction Security is a dependent concept, it is complex and seamless in nature, it needs to be defined under specific circumstances and precise condition or it is meaningless unless it is defined under relational mode with a major concept [as power and peace]. As per Kraus & Williams, “security is a derivative concept; it is meaningless in itself. To have any meaning, security necessarily presupposes something to be secured; as a realm of study it cannot be self-referential” (1997: ix). Realist considers security as the sub-derivative of power or some of the theorists consider it parallel to power, while liberalists believe that security is the essential element for retaining peace (Javaid & Kamal, 2015: 116-117). -

"Family Motacillidae" with Reference to Pakistan
Journal of Bioresource Management Volume 2 Issue 3 Article 10 Short Report: Description and Distribution of Wagtails "Family Motacillidae" with Reference to Pakistan Nadia Yousuf Bioresource Research Centre, Isalamabad, Pakistan Kainaat William Bioresource Research Centre, Islamabad, Pakistan Madeeha Manzoor Bioresource Research Centre, Islamabad, Pakistan, [email protected] Balqees Khanum Bioresource Research Centre, Islamabad, Pakistan Follow this and additional works at: https://corescholar.libraries.wright.edu/jbm Part of the Biodiversity Commons, and the Biology Commons Recommended Citation Yousuf, N., William, K., Manzoor, M., & Khanum, B. (2015). Short Report: Description and Distribution of Wagtails "Family Motacillidae" with Reference to Pakistan, Journal of Bioresource Management, 2 (3). DOI: 10.35691/JBM.5102.0034 ISSN: 2309-3854 online This Article is brought to you for free and open access by CORE Scholar. It has been accepted for inclusion in Journal of Bioresource Management by an authorized editor of CORE Scholar. For more information, please contact [email protected]. Short Report: Description and Distribution of Wagtails "Family Motacillidae" with Reference to Pakistan © Copyrights of all the papers published in Journal of Bioresource Management are with its publisher, Center for Bioresource Research (CBR) Islamabad, Pakistan. This permits anyone to copy, redistribute, remix, transmit and adapt the work for non-commercial purposes provided the original work and source is appropriately cited. Journal -

Water and Power Resources of West Pakistan
Water and Power Resources PAKISTAN "& of WEST I1158 Public Disclosure Authorized A Study in Sector Planning g' c - J) A N D e XJ ~~~~~~~ S >>)~~~~~TM RHELA AS H M I R Public Disclosure Authorized VISLAMABA > 2 t \ . Public Disclosure Authorized C ,,'_ o / z 'N ~~VOLUME g,_ -THE MAIN REPORT \ < ,pre~lppared by a World Bank Study Group Headed by X f .,/ ~~~PIETER LIEFTINCK t i '_z ~~~A. ROBERT SADOVE Public Disclosure Authorized tt I ~~~~~~~~~Deputy Hlead S n THOMAS-4 C.CREYKE ~~~~< < /r~~~~~~~~~~~trigation and Agr-icultut-e WATER AND POWER RESOURCES OF WEST PAKISTAN A Study in Sector Planning Volume I: The Main Report $10.00 Volume II: The Development of Irrigation and Agriculture $12.50 Volume III: Background and Methodology $ 12.50 $28.50 the set Prepared by a World Bank Study Group Headed by Pieter Lieftinck; A. Robert Sadove, Deputy Head; Thomas C. Creyke, Irrigation and Agriculture. Without doubt, the greatest single co- ordinated development operation in which the World Bank has been involved is the massive program for development of the Indus Basin. This pioneering study is an integral part of that project and is unique both in its conceptualization and its compre- hensiveness. It demonstrates the feasibility of a new and more rigorous approach to resource planning and development and will serve as an indispensible model for engi- neers, economists, and planners for years to come. Focal points of the Study are the Indus River, which runs the length of west Paki- stan, several of its tributaries, and a huge natural underground reservoir. -
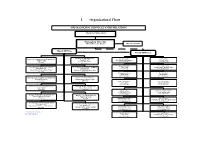
I. Organizational Chart
I. Organizational Chart SBP BANKING SERVICES CORPORATION Board of Directors Managing Director Aftab Mustafa Khan MD Secretariat Head Office Field Offices Personnel Management Currency Management Department Karachi Office Lahore Office Department Javaid Iqbal Taslim Kazi Dr.Muhammad Saleem Amjad Manzoor Chief Manager Chief Manager Director Director Islamabad Office Peshawar Office Foreign Exchange Operations Development Finance Support Tariq Riaz Muhammad Tanwirul Islam Department Department Chief Manager Chief Manager Syed Shahzad Safdar Zaidi Muhammad Mazharul Haq Director Director Rawalpindi Office Quetta Office Asad Shah Ali Hussain Chief Manager Chief Manager * Training & Development Department Accounts Department Amjad Manzoor Muhammad Habib Khan Director* Director Hyderabad Office Faisalabad Office Ali Hussain Sajjad Ali Shah Chief Manager Chief Manager General Services Department Internal Audit Department Zafar Iqbal Maraj Mahmood Director Head Multan Office North Nazimabad Office Javaid Iqbal Marath Ansar Iftikhar Butt Chief Manager Chief Manager Quality Assurance Department Engineering Department Feroza Nabeel Qureshi Fazli Hameed Director Head Muzaffarabad Office Sukkur Office Muhammad Tahir Malik Muhammad Ashraf Khokhar Chief Manager (A) Chief Manager Foreign Exchange Adjudication Department Internal Bank Security Muhammed Saleem Rehmani Department Brig.(R) M. Pervez Akbar Bahawalpur Office Gujranwala Office Director Khadim Hussain Aamir Nazir Bhatti Director Chief Manager (A) Chief Manager (A) * Additional Charge Sialkot Office D.I. Khan Office ( A) Acting Basis Azhar Iqbal Muhammad Humayun Khan Chief Manager Chief Manager Annual Performance Review of SBP BSC FY12 II. Board of Directors S # Name Status 1 Mr. Yaseen Anwar Governor SBP/ Chairman of SBP BSC Board 2 Mr. Abdul Wajid Rana Member/ Principal Officer, Finance Division, GoP 3 Mr. Mirza Qamar Beg Member 4 Mr. -

Pakistan's Institutions
Pakistan’s Institutions: Pakistan’s Pakistan’s Institutions: We Know They Matter, But How Can They We Know They Matter, But How Can They Work Better? Work They But How Can Matter, They Know We Work Better? Edited by Michael Kugelman and Ishrat Husain Pakistan’s Institutions: We Know They Matter, But How Can They Work Better? Edited by Michael Kugelman Ishrat Husain Pakistan’s Institutions: We Know They Matter, But How Can They Work Better? Essays by Madiha Afzal Ishrat Husain Waris Husain Adnan Q. Khan, Asim I. Khwaja, and Tiffany M. Simon Michael Kugelman Mehmood Mandviwalla Ahmed Bilal Mehboob Umar Saif Edited by Michael Kugelman Ishrat Husain ©2018 The Wilson Center www.wilsoncenter.org This publication marks a collaborative effort between the Woodrow Wilson International Center for Scholars’ Asia Program and the Fellowship Fund for Pakistan. www.wilsoncenter.org/program/asia-program fffp.org.pk Asia Program Woodrow Wilson International Center for Scholars One Woodrow Wilson Plaza 1300 Pennsylvania Avenue NW Washington, DC 20004-3027 Cover: Parliament House Islamic Republic of Pakistan, © danishkhan, iStock THE WILSON CENTER, chartered by Congress as the official memorial to President Woodrow Wilson, is the nation’s key nonpartisan policy forum for tackling global issues through independent research and open dialogue to inform actionable ideas for Congress, the Administration, and the broader policy community. Conclusions or opinions expressed in Center publications and programs are those of the authors and speakers and do not necessarily reflect the views of the Center staff, fellows, trustees, advisory groups, or any individuals or organizations that provide financial support to the Center. -

Local Government System in Pakistan
THE LOCAL GOVERNMENT SYSTEM IN paKistan COUNTRY PROFILE 2017–18 PAKISTAN SUMMARY Pakistan is a federal republic with three tiers of government: national, provincial and local. Local government is protected by the constitution in Articles 32 and 140-A, and each province also has its own local-government-enabling legislation and ministries responsible for implementation. District councils and metropolitan corporations are respectively the highest rural and urban tiers of local government in the provinces. Both urban and rural local government have two or three tiers in all provinces except Khyber Pakhtunkhwa, where councils are not identified as either urban or rural. There are 129 district councils across the four provinces, 619 urban councils made up of one city district, four metropolitan corporations, 13 municipal corporations, 96 municipal committees, 148 town councils, 360 urban union committees, and 1,925 rural councils. Additionally there are 3339 neighbourhood, ‘tehsil’ and village councils in Khyber Pakhtunkhwa. Ability to raise local revenue varies according to provincial legislation. District councils and metropolitan corporations have significant responsibilities, often jointly with either higher provincial government – eg for policing (union guards), education, healthcare, roads and local economic development – or with lower levels of local government – eg for water and sanitation, museums and libraries and environmental protection. 1. NATIONAL GOVERNMENT 2. LEGAL BASIS FOR Pakistan is a federal republic with LOCAL GOVERNMENT a bicameral elected parliament 2.1 Constitutional provisions comprising two houses known as the Local government is protected by the 32.1 KEY FACTS Senate and the National Assembly. constitution in Articles 32 and 140-A:32.2a The head of state is the president, who is ■■ Article 32 states: ‘Promotion of local indirectly elected by an electoral college government institutions. -

Usg Humanitarian Assistance to Pakistan in Areas
USG HUMANITARIAN ASSISTANCE TO CONFLICT-AFFECTED POPULATIONS IN PAKISTAN IN FY 2009 AND TO DATE IN FY 2010 Faizabad KEY TAJIKISTAN USAID/OFDA USAID/Pakistan USDA USAID/FFP State/PRM DoD Amu darya AAgriculture and Food Security S Livelihood Recovery PAKISTAN Assistance to Conflict-Affected y Local Food Purchase Populations ELogistics Economic Recovery ChitralChitral Kunar Nutrition Cand Market Systems F Protection r Education G ve Gilgit V ri l Risk Reduction a r Emergency Relief Supplies it a h Shelter and Settlements C e Food For Progress I Title II Food Assistance Shunji gol DHealth Gilgit Humanitarian Coordination JWater, Sanitation, and Hygiene B and Information Management 12/04/09 Indus FAFA N A NWFPNWFP Chilas NWFP AND FATA SEE INSET UpperUpper DirDir SwatSwat U.N. Agencies, E KohistanKohistan Mahmud-e B y Da Raqi NGOs AGCJI F Asadabad Charikar WFP Saidu KUNARKUNAR LowerLower ShanglaShangla BatagramBatagram GoP, NGOs, BajaurBajaur AgencyAgency DirDir Mingora l y VIJaKunar tro Con ImplementingMehtarlam Partners of ne CS A MalakandMalakand PaPa Li Î! MohmandMohmand Kabul Daggar MansehraMansehra UNHCR, ICRC Jalalabad AgencyAgency BunerBuner Ghalanai MardanMardan INDIA GoP e Cha Muzaffarabad Tithwal rsa Mardan dd GoP a a PeshawarPeshawar SwabiSwabi AbbottabadAbbottabad y enc Peshawar Ag Jamrud NowsheraNowshera HaripurHaripur AJKAJK Parachinar ber Khy Attock Punch Sadda OrakzaiOrakzai TribalTribal AreaArea Î! Adj.Adj. PeshawarPeshawar KurrumKurrum AgencyAgency Islamabad Gardez TribalTribal AreaArea AgencyAgency Kohat Adj.Adj. KohatKohat Rawalpindi HanguHangu Kotli AFGHANISTAN KohatKohat ISLAMABADISLAMABAD Thal Mangla reservoir TribalTribal AreaArea AdjacentAdjacent KarakKarak FATAFATA BannuBannu us Bannu Ind " WFP Humanitarian Hub NorthNorth WWaziristanaziristan BannuBannu SOURCE: WFP, 11/30/09 Bhimbar AgencyAgency SwatSwat" TribalTribal AreaArea " Adj.Adj. -

National Highway Authority
tt,rjl \ ' ,--) ''" ,l National Highway Authority FR{$r"un*,vwt$w*u"r'$Yg REQUESTFOR PROPOSAL FOK DETAILED DESIGN trOR DIIALIZATION OF KIJTJZDAR_ CHAMAN SECTIOI\ OF N-25ALONG WITH THE TECHF{ICAI, STUDY & DETAIL DESIGI\ OF ALTERI{ATIVE ROUTE AT LAKPASS & TUI\NEL AT KHOJAK BYPASS June, 2016 I 'l'cohnical l)ctailcdl)csign lbt Dualizal.ionol' Khuzdar - ChamanScction of' N-25 Along With the Study& DctailDcsign ol' r\lLclnatir,el{outc at l-akpess&'l'unncl at Khojah l}y'pass Tableof Contents DESCRIPTION PAGE NO. DetailedDesign for Dualizationof Khuzdar- ChamanSection of N-25 Along With the TechnicalStudy & Detail Designof AlternativeRoute at Lakpass& Tunnelat KhojakBypass APPENDIX (D GOVERNMENT OF PAKISTAII NATIONAL HIGHWAY AUTHORITY 27-MareArea. G-9/1. PostBox No. 120i, ISLAMABAD Dated the Ref No. LETTER OF INVITATION (LOD To, All prospectiveconsultants Gentlemen! We extendwarm welcometo you and invite you for participatingin this project. We hopethat you will live up to your reputationand provideus accurateinformation so that the evaluationis carriedout 'Just and transparent".Please understand that the contentsof this RFP, whereapplicable, shall be deemedpart of the contractagreement. An exampleto this affectcan be the contentsof your work plan and methodologywhich you shall be submittingin your technical proposal.Since that is the basisof the selection,therefore, it shall becomepart of the contract agreementsubject to approval/revisionsof the sameby NHA duringthe negotiations.Similarly, all other servicesand the content contributingto servicesshall -

Peshawar High Court, Peshawar Judicial Department Judgment
JUDGMENT SHEET PESHAWAR HIGH COURT, PESHAWAR JUDICIAL DEPARTMENT C.R No.289-P/2016 JUDGMENT Date of hearing…………30.10.2018....…………….. Petitioner: (Provincial Housing Authority through its Director General): By Mr. Amir Javed, Advocate. Respondent: No.1, Wazir Khan, by Mr. Tariq Khan Hoti, Advocate. Respondent No.4, Maqsad Ali, Girdawar Circle, Kohat in person. Respondent No.5, Farid Khan, Patwari Halqa Jarma, Kohat in person. **** QALANDAR ALI KHAN, J.- This civil revision by Provincial Housing Authority through its Director General (petitioner) is directed against judgments/orders/decrees dated 24.02.2016 by Additional District Judge-IV, Kohat, and also that of the Civil Judge-XI, Kohat, dated 06.09.2014, whereby decree of the latter Court dated 06.09.2014 was maintained by the former/appellate Court; and appeal of the petitioner dismissed vide impugned judgment and decree dated 24.02.2016. 2. The background, forming basis of the instant revision petition, briefly stated, is that originally the Provincial Government was recorded as owner; and Deputy Commissioner, Kohat, in Possession of the 2 land measuring 219 Kanal in Khasra No.1/1122/71 of village Jarma, according to the available record of owners from the year 2003/04. A total of 300 Kanal land, including the said land, was transferred from the Provincial Government to the Prime Minister National Housing Scheme Authority, vide Mutation No.1033 attested on 22.12.1999; but re-transferred to the Provincial Government from the Pakistan Housing Authority, Works Division, Kohat, vide Mutation No.1062 attested on 28.07.2000. The entire land measuring 300 Kanal , including the land in question measuring 219 Kanal (Banjar Jadeed) was transferred by the Provincial Government to the Provincial Housing Authority) Kohat i.e. -

Download PDF Copy of Political Map of Pakistan 2020
TAJIKISTAN C H I N G I L A 70 J & K G Peshawar ( FINAL STATUS NOT YET ISLAMABAD DETERMINED) I T Quetta Lahore 30 - B 60 GILGIT A Karachi Tropic of Cancer L T 80 S 20 I Karakoram Pass Junagadh & U N Manavadar INDUS RIVER S R A V T T E S Y K I A A O F P A N 1767 1947 1823 W H K N U INDUS RIVER Line of Control PAKISTAN T MUZAFFARABAD H Political K SRINAGAR A INDIAN ILLEGALLY OCCUPIED JAMMU & KASHMIR PESHAWAR Scale 1: 3,000,000 N (DISPUTED TERRITORY - FINAL STATUS TO BE DECIDED IN LINE WITH RELEVANT UNSC RESOLUTIONS) ISLAMABAD A R P E B T Y * H S W K orking Boundary I JHELUM RIVER N INDUS RIVER A LAHORE The red dotted line represents approximately the line of control in Jammu & Kashmir. The state of Jammu & Kashmir and its accession H CHENAB RIVER is yet to be decided through a plebiscite under the relevant United Nations Security Council Resolutions. G Actual boundary in the area where remark FRONTIER UNDEFINED P U N J A B appears, would ultimately be decided by the sovereign authorities VI RIVER F RA concerned after the final settlement of the Jammu & Kashmir dispute. *AJ&K stands for Azad Jammu & Kashmir as defined in the AJK Interim A Constitution Act, 1974. QUETTA N CHENAB RIVER SUTLEJ RIVER A A LEGEND T I Capital of Country . ISLAMABAD S Headquarters; Province . .PESHAWAR D Boundary; International . I INDUS RIVER Boundary; Province . N Boundary; Working . P Line of Control .