Thesis on Friday
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Choosing the Best Marine-Derived Omega-3 Products for Therapeutic Use: an Evaluation of the Evidence
STEVENS POINT, WISCONSIN Technical Report Choosing the Best Marine-derived Omega-3 Products for Therapeutic Use: An Evaluation of the Evidence October 2013 The Point Institute is an independent research organization focused on examining and disseminating information about the use of natural therapeutic options for treating and preventing chronic disease. We provide these technical reports as research summaries only-they are not intended to be used in place of sound medical advice by a licensed health care practitioner. The Point Institute Director: Thomas Guilliams Ph.D. Website: www.pointinstitute.org Email: [email protected] The Point Institute www.pointinstitute.org Choosing the Best Marine-derived Omega-3 Products for Therapeutic Use: An Evaluation of the Evidence There is overwhelming data to recommend a wide range of therapeutic uses for marine-derived omega-3 fatty acids (primarily EPA and DHA). Likewise, there are also an overwhelming number of different forms, sources and ways to deliver these omega-3 fatty acids; which unfortunately, has led to confusion in product selection for both clinician and patient alike. We will walk through the most common issues discussed in selecting the appropriate omega-3 fatty acid product, with the goal to bring clarity to the decision-making process. For the most part, the marine omega-3 fatty acid category is dominated by products that can be best described as “fish oil.” That is, while there are products available that deliver omega-3 fatty acids from other marine sources, nearly all the available research has been done with fish oil derived fatty acids. This fish oil data has become the benchmark for efficacy and safety, and is the standard to which we compare throughout this paper. -

Our Nation's Fisheries Will Be a Lot Easier Once We've Used up Everything Except Jellyfish!
Mismanaging Our Nation’s Fisheriesa menu of what's missing Limited quantity: get ‘em while supplies last Ted Stevens Alaskan Surprise Due to years of overfishing, we probably won’t be serving up Pacific Ocean perch, Tanner crab, Greenland turbot or rougheye rockfish. They may be a little hard to swallow, but Senator Stevens and the North Pacific Council will be sure to offer last minute riders, father and son sweetheart deals, record-breaking quotas, industry-led research, conflicts of interest and anti-trust violations. Meanwhile, fur seals, sea lions and sea otters are going hungry and disappearing fast. Surprise! Pacific Rockfish: See No Fish, Eat No Fish Cowcod, Canary Rockfish and Bocaccio are just three examples of rockfish managed by the Pacific Council that are overfished. As for the exact number of West Coast groundfish that are overfished, who knows? Without surveys to tell them what’s going on, what are they managing exactly? Striped Bass: Thin Is In! This popular Atlantic rockfish is available in abundance. Unfortunately, many appear to be undernourished and suffering from lesions – a condition that may point to Omega Protein’s industrial fishery of menhaden, the striper’s favorite prey species. Actually, you may want to hold off on this one until ASMFC starts regulating menhaden. Can you believe there are still no catch limits? Red Snapper Bycatch Platter While we are unable to provide full-size red snapper, we offer this plate of twenty juvenile red snapper discarded as bycatch from a Gulf of Mexico shrimp trawler for your dining pleasure. Shrimpers take and throw away about half of all young red snappers along the Texas coast, so we’ll keep these little guys coming straight from the back of the boat to the back of your throat! Caribbean Reef Fish Grab Bag What’s for dinner from the Caribbean? Who knows? With coral reefs in their jurisdiction, you would expect the Caribbean Council to be pioneering the ecosystem-based management approach and implementing the precautionary principle approach. -

Belonidae Bonaparte 1832 Needlefishes
ISSN 1545-150X California Academy of Sciences A N N O T A T E D C H E C K L I S T S O F F I S H E S Number 16 September 2003 Family Belonidae Bonaparte 1832 needlefishes By Bruce B. Collette National Marine Fisheries Service Systematics Laboratory National Museum of Natural History, Washington, DC 20560–0153, U.S.A. email: [email protected] Needlefishes are a relatively small family of beloniform fishes (Rosen and Parenti 1981 [ref. 5538], Collette et al. 1984 [ref. 11422]) that differ from other members of the order in having both the upper and the lower jaws extended into long beaks filled with sharp teeth (except in the neotenic Belonion), the third pair of upper pharyngeal bones separate, scales on the body relatively small, and no finlets following the dorsal and anal fins. The nostrils lie in a pit anterior to the eyes. There are no spines in the fins. The dorsal fin, with 11–43 rays, and anal fin, with 12–39 rays, are posterior in position; the pelvic fins, with 6 soft rays, are located in an abdominal position; and the pectoral fins are short, with 5–15 rays. The lateral line runs down from the pectoral fin origin and then along the ventral margin of the body. The scales are small, cycloid, and easily detached. Precaudal vertebrae number 33–65, caudal vertebrae 19–41, and total verte- brae 52–97. Some freshwater needlefishes reach only 6 or 7 cm (2.5 or 2.75 in) in total length while some marine species may attain 2 m (6.5 ft). -

Studies on Blood Proteins in Herring
FiskDir. Skr. SET.HavUi~ders., 15: 356-367. COMPARISON OF PACIFIC SARDINE AND ATLANTIC MENHADEN FISHERIES BY JOHN L. MCHUGH Office of Marine Resources U.S. Department of the Interior, Washington, D.C. INTRODUCTION The rise and fall of the North American Pacific coast sardine fishery is well known. Oilce the most important fishery in the western hemisphere in weight of fish landed, it now produces virtually nothing. The meal and oil industry based on the Pacific sardine (Sardinoljs caerulea) resource no longer exists. The sardine fishery (Fig. 1A) began in 1915, rose fairly steadily to its peak in 1936 (wit11 a dip during the depression), maintained an average annual catch of more than 500 000 tons until 1944, then fell off sharply. Annual production has not exceeded 100 000 tons since 1951, and commercial sardine fishing now is prohibited in California waters. A much smaller fishery for the southern sub-population developed off Baja California in 195 1. The decline of the west coast sardine fishery gave impetus to the much older menhaden (Breuoortia tyranlzus) fishery (Fig. 1B) along the Atlantic coast of the United States. Fishing for Atlantic menhaden began early in the nineteenth century. From the 1880's until the middle 1930's the annual catch varied around about 200 000 tons. In the late 1930's annual landings began to increase and from 1953 to 1962 inclusive re- mained above 500 000 tons. The peak year was 1956, with a catch of nearly 800 000 tons. After 1962 the catch began to fall off sharply, reach- ing a low of less than 250 000 tons in 1967. -

Sharkcam Fishes
SharkCam Fishes A Guide to Nekton at Frying Pan Tower By Erin J. Burge, Christopher E. O’Brien, and jon-newbie 1 Table of Contents Identification Images Species Profiles Additional Info Index Trevor Mendelow, designer of SharkCam, on August 31, 2014, the day of the original SharkCam installation. SharkCam Fishes. A Guide to Nekton at Frying Pan Tower. 5th edition by Erin J. Burge, Christopher E. O’Brien, and jon-newbie is licensed under the Creative Commons Attribution-Noncommercial 4.0 International License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc/4.0/. For questions related to this guide or its usage contact Erin Burge. The suggested citation for this guide is: Burge EJ, CE O’Brien and jon-newbie. 2020. SharkCam Fishes. A Guide to Nekton at Frying Pan Tower. 5th edition. Los Angeles: Explore.org Ocean Frontiers. 201 pp. Available online http://explore.org/live-cams/player/shark-cam. Guide version 5.0. 24 February 2020. 2 Table of Contents Identification Images Species Profiles Additional Info Index TABLE OF CONTENTS SILVERY FISHES (23) ........................... 47 African Pompano ......................................... 48 FOREWORD AND INTRODUCTION .............. 6 Crevalle Jack ................................................. 49 IDENTIFICATION IMAGES ...................... 10 Permit .......................................................... 50 Sharks and Rays ........................................ 10 Almaco Jack ................................................. 51 Illustrations of SharkCam -

Atlantic Cod (Gadus Morhua) Off Newfoundland and Labrador Determined from Genetic Variation
COSEWIC Assessment and Update Status Report on the Atlantic Cod Gadus morhua Newfoundland and Labrador population Laurentian North population Maritimes population Arctic population in Canada Newfoundland and Labrador population - Endangered Laurentian North population - Threatened Maritimes population - Special Concern Arctic population - Special Concern 2003 COSEWIC COSEPAC COMMITTEE ON THE STATUS OF COMITÉ SUR LA SITUATION ENDANGERED WILDLIFE DES ESPÈCES EN PÉRIL IN CANADA AU CANADA COSEWIC status reports are working documents used in assigning the status of wildlife species suspected of being at risk. This report may be cited as follows: COSEWIC 2003. COSEWIC assessment and update status report on the Atlantic cod Gadus morhua in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. xi + 76 pp. Production note: COSEWIC would like to acknowledge Jeffrey A. Hutchings for writing the update status report on the Atlantic cod Gadus morhua, prepared under contract with Environment Canada. For additional copies contact: COSEWIC Secretariat c/o Canadian Wildlife Service Environment Canada Ottawa, ON K1A 0H3 Tel.: (819) 997-4991 / (819) 953-3215 Fax: (819) 994-3684 E-mail: COSEWIC/[email protected] http://www.cosewic.gc.ca Également disponible en français sous le titre Rapport du COSEPAC sur la situation de la morue franche (Gadus morhua) au Canada Cover illustration: Atlantic Cod — Line drawing of Atlantic cod Gadus morhua by H.L. Todd. Image reproduced with permission from the Smithsonian Institution, NMNH, Division of Fishes. Her Majesty the Queen in Right of Canada, 2003 Catalogue No.CW69-14/311-2003-IN ISBN 0-662-34309-3 Recycled paper COSEWIC Assessment Summary Assessment summary — May 2003 Common name Atlantic cod (Newfoundland and Labrador population) Scientific name Gadus morhua Status Endangered Reason for designation Cod in the inshore and offshore waters of Labrador and northeastern Newfoundland, including Grand Bank, having declined 97% since the early 1970s and more than 99% since the early 1960s, are now at historically low levels. -

Appendix A. Species List and Threatened Or Endangered Species
Appendix A Great egrets rely on wetlands for feeding and nesting. © Mark Wilson Species Lists and Threatened or Endangered Species ■ Bird Species of the Complex ■ Mammal Species of the Complex ■ Reptile and Amphibian Species of the Complex ■ Fish Species of the Complex ■ Butterfly Species of the Complex ■ Threatened or Endanged Species Appendix A Bird Species of the Complex Conscience Lido Oyster Target Bird Species Amagansett Morton Sayville Seatuck Wertheim Point Beach Bay Rock s=Spring (Mar–May) S=Summer (Jun–Aug) A=Autumn (Sep–Nov) W=Winter (Dec–Feb) *=Birds documented breeding at the Complex Red-Throated Loon s AW s AW s AW s AW s AW s AW s AW s AW Gavia stellata Common Loon (Sc) s AW s AW s AW sSAW s AW s AW s AW sSAW Gavia immer Horned Grebe s AW s AW s AW s AW s AW s AW s AW s AW Podiceps auritus Red Necked Grebe s AW s AW Podiceps grisegena Eared Grebe s AW Podiceps nigricollis Pied-billed Grebe*(St) s AW s AW s AW s AW sSAW sSAW sSAW* Podilymbus podiceps Great Cormorant s AW s AW s AW s AW s AW s AW s AW s AW Phalacrocorax carbo Double-crested Cormorant sSAW sSAW sSAW sSAW sSAW sSAW sSAW sSAW Phalacrocorax auritus Brown Pelican S S Pelecanus occidentalis Northern Gannet s AW s AW s AW s AW s AW Morus bassanus Brown Booby S Sula leucogaster American Bittern* (Sc) s AW s AW s AW s AW sSAW* s AW sSAW* Botaurus lentiginosus Least Bittern*(St) sSA* sSAW* Ixobrychus exilis Great Blue Heron s AW sSAW sSAW sSAW sSAW sSAW sSAW sSAW Ardea herodias Great Egret sSA sSA sSAW sSA sSA sSA sSA sSAW Casmerodius albus Snowy Egret sSA sSA sSA sSA -
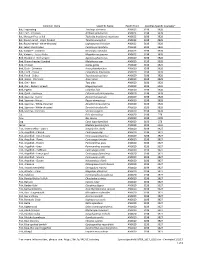
*For More Information, Please See
Common Name Scientific Name Health Point Specifies-Specific Course(s)* Bat, Frog-eating Trachops cirrhosus AN0023 3198 3928 Bat, Fruit - Jamaican Artibeus jamaicensis AN0023 3198 3928 Bat, Mexican Free-tailed Tadarida brasiliensis mexicana AN0023 3198 3928 Bat, Round-eared - stripe-headed Tonatia saurophila AN0023 3198 3928 Bat, Round-eared - white-throated Lophostoma silvicolum AN0023 3198 3928 Bat, Seba's short-tailed Carollia perspicillata AN0023 3198 3928 Bat, Vampire - Common Desmodus rotundus AN0023 3198 3928 Bat, Vampire - Lesser False Megaderma spasma AN0023 3198 3928 Bird, Blackbird - Red-winged Agelaius phoeniceus AN0020 3198 3928 Bird, Brown-headed Cowbird Molothurus ater AN0020 3198 3928 Bird, Chicken Gallus gallus AN0020 3198 3529 Bird, Duck - Domestic Anas platyrhynchos AN0020 3198 3928 Bird, Finch - House Carpodacus mexicanus AN0020 3198 3928 Bird, Finch - Zebra Taeniopygia guttata AN0020 3198 3928 Bird, Goose - Domestic Anser anser AN0020 3198 3928 Bird, Owl - Barn Tyto alba AN0020 3198 3928 Bird, Owl - Eastern Screech Megascops asio AN0020 3198 3928 Bird, Pigeon Columba livia AN0020 3198 3928 Bird, Quail - Japanese Coturnix coturnix japonica AN0020 3198 3928 Bird, Sparrow - Harris' Zonotrichia querula AN0020 3198 3928 Bird, Sparrow - House Passer domesticus AN0020 3198 3928 Bird, Sparrow - White-crowned Zonotrichia leucophrys AN0020 3198 3928 Bird, Sparrow - White-throated Zonotrichia albicollis AN0020 3198 3928 Bird, Starling - Common Sturnus vulgaris AN0020 3198 3928 Cat Felis domesticus AN0020 3198 279 Cow Bos taurus -
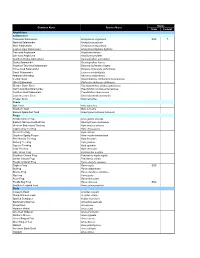
St. Joseph Bay Native Species List
Status Common Name Species Name State Federal Amphibians Salamanders Flatwoods Salamander Ambystoma cingulatum SSC T Marbled Salamander Ambystoma opacum Mole Salamander Ambystoma talpoideum Eastern Tiger Salamander Ambystoma tigrinum tigrinum Two-toed Amphiuma Amphiuma means One-toed Amphiuma Amphiuma pholeter Southern Dusky Salamander Desmognathus auriculatus Dusky Salamander Desmognathus fuscus Southern Two-lined Salamander Eurycea bislineata cirrigera Three-lined Salamander Eurycea longicauda guttolineata Dwarf Salamander Eurycea quadridigitata Alabama Waterdog Necturus alabamensis Central Newt Notophthalmus viridescens louisianensis Slimy Salamander Plethodon glutinosus glutinosus Slender Dwarf Siren Pseudobranchus striatus spheniscus Gulf Coast Mud Salamander Pseudotriton montanus flavissimus Southern Red Salamander Pseudotriton ruber vioscai Eastern Lesser Siren Siren intermedia intermedia Greater Siren Siren lacertina Toads Oak Toad Bufo quercicus Southern Toad Bufo terrestris Eastern Spadefoot Toad Scaphiopus holbrooki holbrooki Frogs Florida Cricket Frog Acris gryllus dorsalis Eastern Narrow-mouthed Frog Gastrophryne carolinensis Western Bird-voiced Treefrog Hyla avivoca avivoca Cope's Gray Treefrog Hyla chrysoscelis Green Treefrog Hyla cinerea Southern Spring Peeper Hyla crucifer bartramiana Pine Woods Treefrog Hyla femoralis Barking Treefrog Hyla gratiosa Squirrel Treefrog Hyla squirella Gray Treefrog Hyla versicolor Little Grass Frog Limnaoedus ocularis Southern Chorus Frog Pseudacris nigrita nigrita Ornate Chorus Frog Pseudacris -

Status of Shortnose Sturgeon in the Potomac River
Final Report Status of Shortnose Sturgeon in the Potomac River PART I – FIELD STUDIES Report prepared by: Boyd Kynard (Principal Investigator) Matthew Breece and Megan Atcheson (Project Leaders) Micah Kieffer (Co-Investigator) U. S. Geological Survey, Biological Resources Division Leetown Science Center S. O. Conte Anadromous Fish Research Center Turners Falls, Massachusetts 01376 and Mike Mangold (Assistant Project Leader) U. S. Fish and Wildlife Service, Maryland Fishery Resources Office 177 Admiral Cochrane Drive Annapolis, Maryland 21401 Report prepared for: National Park Service National Capital Region Washington, D.C USGS Natural Resources Preservation Project E 2002-7 NPS Project Coordinator – Jim Sherald BRD Project Coordinator – Ed Pendelton July 20, 2007 Gravid shortnose sturgeon female captured at river kilometer 63 on the Potomac River. Project leader, Matthew Breece (USGS), is shown with the fish on March 23, 2006. Summary Field studies during more than 3 years (March 2004–July 2007) collected data on life history of Potomac River shortnose sturgeon Acipenser brevirostrum to understand their biological status in the river. We sampled intensively for adults using gill nets, but captured only one adult in 2005. Another adult was captured in 2006 by a commercial fisher. Both fish were females with excellent body and fin condition, both had mature eggs, and both were telemetry- tagged to track their movements. The lack of capturing adults, even when intensive netting was guided by movements of tracked fish, indicated abundance of the species was less than in any river known with a sustaining population of the species. Telemetry tracking of the two females (one during September 2005–July 2007, one during March 2006–February 2007) found they remained in the river for all the year, not for just a few months like sturgeons on a coastal migration. -

Little Fish, Big Impact: Managing a Crucial Link in Ocean Food Webs
little fish BIG IMPACT Managing a crucial link in ocean food webs A report from the Lenfest Forage Fish Task Force The Lenfest Ocean Program invests in scientific research on the environmental, economic, and social impacts of fishing, fisheries management, and aquaculture. Supported research projects result in peer-reviewed publications in leading scientific journals. The Program works with the scientists to ensure that research results are delivered effectively to decision makers and the public, who can take action based on the findings. The program was established in 2004 by the Lenfest Foundation and is managed by the Pew Charitable Trusts (www.lenfestocean.org, Twitter handle: @LenfestOcean). The Institute for Ocean Conservation Science (IOCS) is part of the Stony Brook University School of Marine and Atmospheric Sciences. It is dedicated to advancing ocean conservation through science. IOCS conducts world-class scientific research that increases knowledge about critical threats to oceans and their inhabitants, provides the foundation for smarter ocean policy, and establishes new frameworks for improved ocean conservation. Suggested citation: Pikitch, E., Boersma, P.D., Boyd, I.L., Conover, D.O., Cury, P., Essington, T., Heppell, S.S., Houde, E.D., Mangel, M., Pauly, D., Plagányi, É., Sainsbury, K., and Steneck, R.S. 2012. Little Fish, Big Impact: Managing a Crucial Link in Ocean Food Webs. Lenfest Ocean Program. Washington, DC. 108 pp. Cover photo illustration: shoal of forage fish (center), surrounded by (clockwise from top), humpback whale, Cape gannet, Steller sea lions, Atlantic puffins, sardines and black-legged kittiwake. Credits Cover (center) and title page: © Jason Pickering/SeaPics.com Banner, pages ii–1: © Brandon Cole Design: Janin/Cliff Design Inc. -

Variability in Trawl Survey Catches of Pacific Ocean Perch, Shortraker Rockfish, and Rougheye Rockfish in the Gulf of Alaska David M
Biology, Assessment, and Management of North Pacific Rockfishes 411 Alaska Sea Grant College Program • AK-SG-07-01, 2007 Variability in Trawl Survey Catches of Pacific Ocean Perch, Shortraker Rockfish, and Rougheye Rockfish in the Gulf of Alaska David M. Clausen and Jeffrey T. Fujioka National Marine Fisheries Service, Auke Bay Laboratory, Juneau, Alaska Abstract Little information is available on the comparative variability of Pacific ocean perch, shortraker rockfish, and rougheye rockfish catches in Alaska trawl surveys. In this study, data were first examined for these three species from three localized experimental bottom trawl surveys in the Gulf of Alaska in 1993, 1998, and 1999 to compare the variability of catch rates for each species. When catch rates were analyzed within favorable fishing areas for each species, the coefficients of variation for Pacific ocean perch were approximately 2.5 to 3 times greater than those of shortraker or rougheye rockfish, indicating that the latter two species are much more even in their distribution. To provide a broader comparison of variability, catch rates of the three species were also examined from seven large-scale bottom trawl surveys conducted in the Gulf of Alaska from 1984 to 2001. Using geographic information systems software, subsets of the hauls in these surveys were selected in what were determined to be favorable fishing areas for either Pacific ocean perch or for shortraker and rougheye rockfish. Analysis of the species’ catch rates in these hauls indicated results that were very simi- lar to those of the experimental trawl surveys; the variability of Pacific ocean perch was 2.1-2.3 times greater than that for either shortraker or rougheye rockfish.