Study on Compounds Analysis and Anti Colorectal Cancer E Cacy Of
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
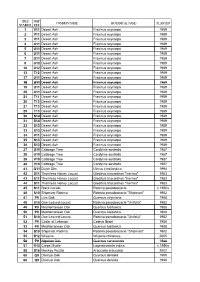
World Collection Tree List: by Tree Number
TREE MAP COMMON NAME BOTANICAL NAME PLANTED NUMBER REF 1 V11 Desert Ash Fraxinus oxycarpa 1989 2 V11 Desert Ash Fraxinus oxycarpa 1989 3 V11 Desert Ash Fraxinus oxycarpa 1989 4 U11 Desert Ash Fraxinus oxycarpa 1989 5 U11 Desert Ash Fraxinus oxycarpayp 1989 6 U11 Desert Ash Fraxinus oxycarpa 1989 7 U11 Desert Ash Fraxinus oxycarpa 1989 8 U12 Desert Ash Fraxinus oxycarpa 1989 10 U12 Desert Ash Fraxinus oxycarpa 1989 13 T12 Desert Ash Fraxinus oxycarpa 1989 17 U11 Desert Ash Fraxinus oxycarpa 1989 18 U11 Desert Ash Fraxinus oxycarpa 1989 19 U11 Desert Ash Fraxinus oxycarpa 1989 20 U11 Desert Ash Fraxinus oxycarpa 1989 21 T11 Desert Ash Fraxinus oxycarpa 1989 26 T13 Desert Ash Fraxinus oxycarpa 1989 27 T13 Desert Ash Fraxinus oxycarpa 1989 29 T13 Desert Ash Fraxinus oxycarpa 1989 30 S14 Desert Ash Fraxinus oxycarpa 1989 31 S14 Desert Ash Fraxinus oxycarpa 1989 32 S13 Desert Ash Fraxinus oxycarpa 1989 33 S13 Desert Ash Fraxinus oxycarpa 1989 34 V11 Desert Ash Fraxinus oxycarpa 1989 35 S13 Desert Ash Fraxinus oxycarpa 1989 36 S13 Desertese t Ash s Fraxinusa us oox ycaycar papa 1989989 37 U19 Cabbage Tree Cordyline australis 1987 38 U19 Cabbage Tree Cordyline australis 1987 39 V19 Cabbage Tree Cordyline australis 1987 40 V19 Cabbage Tree Cordyline australis 1987 41 Q11 Dutch Elm Ulmus x hollandica 1994 42 S11 Thornless Honey Locust Gleditsia triacanthos "Inermis" 1983 43 S11 Thornless HoneyHoney LocustLocust Gleditsia triacanthos ""Inermis"Inermis" 1983 44 S11 Thornless Honey Locust Gleditsia triacanthos "Inermis" 1983 45 S11 Black Locust Robinia -

Gallnuts: a Potential Treasure in Anticancer Drug Discovery
Hindawi Evidence-Based Complementary and Alternative Medicine Volume 2018, Article ID 4930371, 9 pages https://doi.org/10.1155/2018/4930371 Review Article Gallnuts: A Potential Treasure in Anticancer Drug Discovery Jiayu Gao ,1 Xiao Yang ,2 Weiping Yin ,1 and Ming Li3 1 School of Chemical and Pharmaceutical Engineering, Henan University of Scientifc and Technology, Henan, China 2School of Clinical Medicine, Henan University of Scientifc and Technology, Henan, China 3Luoyang Traditional Chinese Medicine Association, Luoyang, Henan, China Correspondence should be addressed to Jiayu Gao; [email protected] Received 8 September 2017; Revised 17 February 2018; Accepted 21 February 2018; Published 29 March 2018 Academic Editor: Chris Zaslawski Copyright © 2018 Jiayu Gao et al. Tis is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Introduction. In the discovery of more potent and selective anticancer drugs, the research continually expands and explores new bioactive metabolites coming from diferent natural sources. Gallnuts are a group of very special natural products formed through parasitic interaction between plants and insects. Tough it has been traditionally used as a source of drugs for the treatment of cancerous diseases in traditional and folk medicinal systems through centuries, the anticancer properties of gallnuts are barely systematically reviewed. Objective. To evidence the traditional uses and phytochemicals and pharmacological mechanisms in anticancer aspects of gallnuts, a literature review was performed. Materials and Methods. Te systematic review approach consisted of searching web-based scientifc databases including PubMed, Web of Science, and Science Direct. -

Museum of Economic Botany, Kew. Specimens Distributed 1901 - 1990
Museum of Economic Botany, Kew. Specimens distributed 1901 - 1990 Page 1 - https://biodiversitylibrary.org/page/57407494 15 July 1901 Dr T Johnson FLS, Science and Art Museum, Dublin Two cases containing the following:- Ackd 20.7.01 1. Wood of Chloroxylon swietenia, Godaveri (2 pieces) Paris Exibition 1900 2. Wood of Chloroxylon swietenia, Godaveri (2 pieces) Paris Exibition 1900 3. Wood of Melia indica, Anantapur, Paris Exhibition 1900 4. Wood of Anogeissus acuminata, Ganjam, Paris Exhibition 1900 5. Wood of Xylia dolabriformis, Godaveri, Paris Exhibition 1900 6. Wood of Pterocarpus Marsupium, Kistna, Paris Exhibition 1900 7. Wood of Lagerstremia parviflora, Godaveri, Paris Exhibition 1900 8. Wood of Anogeissus latifolia , Godaveri, Paris Exhibition 1900 9. Wood of Gyrocarpus jacquini, Kistna, Paris Exhibition 1900 10. Wood of Acrocarpus fraxinifolium, Nilgiris, Paris Exhibition 1900 11. Wood of Ulmus integrifolia, Nilgiris, Paris Exhibition 1900 12. Wood of Phyllanthus emblica, Assam, Paris Exhibition 1900 13. Wood of Adina cordifolia, Godaveri, Paris Exhibition 1900 14. Wood of Melia indica, Anantapur, Paris Exhibition 1900 15. Wood of Cedrela toona, Nilgiris, Paris Exhibition 1900 16. Wood of Premna bengalensis, Assam, Paris Exhibition 1900 17. Wood of Artocarpus chaplasha, Assam, Paris Exhibition 1900 18. Wood of Artocarpus integrifolia, Nilgiris, Paris Exhibition 1900 19. Wood of Ulmus wallichiana, N. India, Paris Exhibition 1900 20. Wood of Diospyros kurzii , India, Paris Exhibition 1900 21. Wood of Hardwickia binata, Kistna, Paris Exhibition 1900 22. Flowers of Heterotheca inuloides, Mexico, Paris Exhibition 1900 23. Leaves of Datura Stramonium, Paris Exhibition 1900 24. Plant of Mentha viridis, Paris Exhibition 1900 25. Plant of Monsonia ovata, S. -

The Wood Cross Sections of Hermann Nördlinger (1818–1897)
IAWA Journal, Vol. 29 (4), 2008: 439–457 THE WOOD CROSS SECTIONS OF HERMANN NÖRDLINGER (1818–1897) Ben Bubner Leibniz-Zentrum für Agrarlandschaftsforschung (ZALF) e.V., Institut für Landschaftsstoffdynamik, Eberswalder Str. 84, 15374 Müncheberg, Germany [E-mail: [email protected]] SUMMARY Hermann Nördlinger (1818–1897), forestry professor in Hohenheim, Germany, published a series of wood cross sections in the years 1852 to 1888 that are introduced here to the modern wood anatomist. The sec- tions, which vary from 50 to 100 μm in thickness, are mounted on sheets of paper and their quality is high enough to observe microscopic details. Their technical perfection is as remarkable as the mode of distribution: sections of 100 wood species were presented in a box together with a booklet containing wood anatomical descriptions. These boxes were dis- tributed as books by the publisher Cotta, from Stuttgart, Germany, with a maximum circulation of 500 per volume. Eleven volumes comprise 1100 wood species from all over the world. These include not only conifers and broadleaved trees but also shrubs, ferns and palms representing a wide variety of woody structures. Excerpts of this collection were also pub- lished in Russian, English and French. Today, volumes of Nördlingerʼs cross sections are found in libraries throughout Europe and the United States. Thus, they are relatively easily accessible to wood anatomists who are interested in historic wood sections. A checklist with the content of each volume is appended. Key words: Cross section, wood collection, wood anatomy, history. INTRODUCTION Wood scientists who want to distinguish wood species anatomically rely on thin sec- tions mounted on glass slides and descriptions in books that are illustrated with micro- photographs. -

1 Trees: Structure and Function Vol 31: 1571-1581, 2017
Biotechnological efforts for the propagation of Quercus lusitanica Lam., an endangered species Trees: Structure and Function vol 31: 1571-1581, 2017 San José MC, Martínez MT, Cernadas MJ, Montenegro R, Mosteiro F, Corredoira E Group of Biotechnology and Forestry Improvement. Instituto de Investigaciones Agrobiológicas de Galicia. CSIC. Avda. de Vigo s/n. 15705 Santiago de Compostela, Spain. E-mail: [email protected] Abstract This is the first time that a simple protocol is presented for the micropropagation and short-term conservation of Quercus lusitanica, an endemic and endangered species that is found in very restricted habitats of the Iberian Peninsula and North Africa. Branch segments from 15-20-year-old trees were forced-flushed, and the forced shoots were used as a source of explants for culture initiation. The best multiplication rates were obtained on Woody Plant Medium (WPM) supplemented with 0.1 mg l-1 benzyladenine for the genotype CDL, or 0.2 mg l-1 benzyladenine, 0.5 mg l-1 indole acetic acid and 0.2 mg l-1 zeatin in the case of the genotype SC1. Although the cultivation of shoots in the horizontal position improved the proliferation rates, the shoot vitrification was increased under these conditions. The best rooting rates were obtained on half-strength WPM supplemented with 3 mg l-1 indole-3-butyric acid for 7 days with subsequent transfer to auxin-free medium supplemented with 0.4% activated charcoal. An initial 5-day dark period was detrimental to the quality of regenerated plantlets. Well-acclimatized plantlets were transferred to the field with a 70% survival rate. -

Querucs Lusitanica
Design Standards for Urban Infrastructure Plant Species for Urban Landscape Projects in Canberra Botanical Name: Quercus lusitanica (Qlu) Common Name: Lusitanian oak, Portuguese oak, Gall oak Species Description • Semi-evergreen in warmer climates • Wide spreading tree • Deeply fissured brownish-grey bark. • Glossy dark green to grey/green leaves. Distinguished from Quercus robur by regular and less deeply lobed leaves. • Later autumn colour and leaf shedding than 16m other oaks • Flowers are catkins which appear in spring • Plentiful acorns Height and width 16 metres tall by 12 metres wide Species origin Morocco, Portugal and Galicia, Spain 12m Landscape use • Available Soil Volume required: ≥45m3 • Excellent shade tree • Suitable for use as a street tree and in parks or open areas where solar access in winter is not an issue as it retains leaves to mid-winter Use considerations • Has performed very well in Canberra • High frost and drought tolerance • Prefers well-drained deep fertile loams but grows in a range of soils across Canberra and withstands dry periods better than Quercus robur • Grows best in full sun but tolerates shade when young • Long lived • Slow growing but hardy and persistent • Low flammability • Acorn drop may be a nuisance in pedestrian areas • Produces pollen Examples in Canberra Forbes St, Turner and a group of trees in Booroondara Street, Reid on the ACT Tree Register Availability Commercially available but highly variable; suitable stock will need to be specifically propagated from Canberra provenance . -

Place Orders by Phone Or Email [email protected]
Place orders by phone or email [email protected] 0417 535 917 / 5628 1507 Pick up or delivery available Stock available from July 1st Bare Root Seedling Order Form Winter 2019 Page 1/2 Price per tree* Quantity *Minimum order of 10 per variety Acer beurgerianum - Trident Maple $ 3.00 Acer campestre - Hedge Maple $ 3.00 Acer davidii ~ David's maple $ 3.00 Acer freemanii - Jeffersred maple $ 4.50 Acer negundo ~ Box maple $ 3.00 Acer palmatum ~ Japanese maple $ 3.00 Acer pseudoplatanus ~ Sycamore maple $ 3.00 Acer rubrum ~ Red maple $ 4.50 Acer saccharinum ~ Silver maple $ 4.00 Aesculus hippocastanum ~ English horse chestnut $ 3.00 Aesculus indica ~ Indian horse chestnut $ 3.00 Albizia julibrissin ~ Pink silk tree $ 4.00 Alnus cordata ~ Italian elder $ 3.00 Betula nigra ~ River birch $ 4.00 Betula pendula alba ~ Silver birch $ 3.00 Betula platyphylla japonica ~ Asian white birch $ 4.00 Catalpa bignoniodies ~ Southern catalpa $ 3.00 Cedrus deodara - Himalayan cedar $ 4.00 Sold out Celtis australis ~ European nettle $ 3.00 Cercis chinensis ~ Chinease redbud $ 4.00 Cercis siliquastrum ~ Judas tree $ 4.00 Cornus capitata - Himalayan flowering dogwood $ 3.00 Cornus florida ~ Flowering dogwood $ 4.00 Cotinus coggygria purpurea ~ Purple smoke bush $ 4.00 Sold out Fagus sylvatica ~ European beech $ 6.00 Fagus sylvatica atropurpuream ~ Copper beech $ 6.00 Sold out Fraxinus americana ~ White ash $ 3.00 Fraxinus excelsior ~ European ash $ 3.00 Fraxinus ornus ~ Flowering ash $ 3.00 Fraxinus oxycarpa ~ Desert ash $ 3.00 Fraxinus pennsylvanica ~ Green -

Overcoming Sexual Sterility in Conservation of Endangered Species
Plant Cell, Tissue and Organ Culture (PCTOC) https://doi.org/10.1007/s11240-019-01558-x ORIGINAL ARTICLE Overcoming sexual sterility in conservation of endangered species: the prominent role of biotechnology in the multiplication of Zelkova sicula (Ulmaceae), a relict tree at the brink of extinction Angela Carra1 · Caterina Catalano1 · Ornella Badalamenti1 · Francesco Carimi1 · Salvatore Pasta1,2 · Antonio Motisi1 · Loredana Abbate1 · Francesca La Bella1 · Laurence Fazan2 · Gregor Kozlowski2,3 · Giuseppe Garfì1 Received: 13 September 2018 / Accepted: 7 January 2019 © Springer Nature B.V. 2019 Abstract Biotechnology provides valuable tools to support conservation of plant species, especially in case of threatened taxa or when dealing with seed unavailability, low viability or sterility. However, plant cell culture methods have often to face prob- lems associated with tissue recalcitrance to in vitro systems. Recalcitrance can be related to a variety of triggering factors, involving many efforts and manipulations within one or more of the micropropagation stages before obtaining successful results. An in vitro propagation protocol was developed for Zelkova sicula, a very rare and endangered relict tree, endemic to Sicily (Southern Italy). The species revealed extremely recalcitrant to in vitro culture approaches, but after many trials throughout a number of years an effective micropropagation protocol was completed. The rooting rate was about 84% of the treated explants, 8% of which were successfully acclimatized outdoor and reintroduced in the wild within a comprehensive conservation project. The technique allowed to overcome the problems of sexual sterility of this species, hence contributing concretely to contrast the problems connected with its conservation. However, additional efforts need to be carried out in order to refine the acclimatization step and further improve the whole process effectiveness. -

Mistletoes on Mmahgh J) Introduced Trees of the World Agriculture
mmAHGH J) Mistletoes on Introduced Trees of the World Agriculture Handbook No. 469 Forest Service U.S. Department of Agriculture Mistletoes on Introduced Trees of the World by Frank G. Hawksworth Plant Pathologist Rocky Mountain Forest and Range Experiment Station Agriculture Handbook No. 469 Forest Service U.S. Department of Agriculture December 1974 CONTENTS Page Introduction ^ Mistletoes and Hosts 3 Host Index of Mistletoes 27 Literature Cited ^^ Library of Congress Catalog No. 74-600182 For sale by the Superintendent of Documents, U.S. Government Printing Office Washington B.C. 20402 Price 75 cents Stock Number 0100-03303 MISTLETOES ON INTRODUCED TREES OF THE WORLD INTRODUCTION Spaulding (1961) published the first attempt at a worldwide inven- tory of the diseases of foreign (introduced) trees of the world. With the widespread introduction of trees to many parts of the world, it is becoming of increasing importance to know the susceptibility of trees introduced to new disease situations. Spaulding's comprehensive lists included forest tree diseases caused by fungi, bacteria, and viruses, but not the mistletoes. Therefore this publication on the mistletoes was prepared to supplement his work. Spaulding considered only forest trees, but the coverage here is expanded to include mistletoes parasitic on introduced forest, crop, orchard, and ornamental trees. In some instances, mistletoes are reported on trees cultivated within different parts of a country where the tree is native. Such records are included if it is indicated in the publication that the mistletoe in question is on planted trees. "Mistletoe" as used in this paper refers to any member of the fam- ilies Loranthaceae or Viscaceae. -

6. RPA14150001015.Pdf
62 | P a g e International Standard Serial Number (ISSN): 2319-8141 International Journal of Universal Pharmacy and Bio Sciences 3(1): January-February 2014 INTERNATIONAL JOURNAL OF UNIVERSAL PHARMACY AND BIO SCIENCES IMPACT FACTOR 1.89*** ICV 5.13*** Pharmaceutical Sciences RESEARCH ARTICLE……!!! PHARMACOGNOSTICAL AND PHYSICO-CHEMICAL EVALUATION ON MAYAPHALA GRANULES– AN AYURVEDIC FORMULATION 1Bhavbhushan, 2Mandip Goyal, 3Harisha C.R, 4V.J.Shukla, 5Chandola H.M. 1P G Scholar, Department of Kayachikitsha, 2Assistant professor, Department of Kayachikitsha, 3 Head, Pharmacognosy Laboratory, 4Head, Pharmaceutical chemistry Laboratory, IPGT & RA, Gujarat Ayurved University, Jamnagar, Gujarat, India, 5Director, Chaudhary Brahm Prakash Ayurved Charak Sansthan, Khera Dabar, Najafgarh, New Delhi. ABSTRACT KEYWORDS: Quercus infectoria Olivier. Commonly known as “Mayaphala” in Sanskrit, is most untouched and unexplored drug till today by most Mayaphala, Quercus infectoria Olivier of the researchers. Since the plant is reported for many biological .Pharmacognosy, activities, it has been selected for detailed Pharmacognostical study Phytochemistry of its Gall granules including Physico-chemical analysis following For Correspondence: standard guidelines of Ayurvedic Pharmacopoeia of India. The Bhavbhushan * Address: PG Scholar, microscopic characteristics of granules shows prismatic crystals of Department of calcium oxalate, large quantity of tannin materials, oil globules, Kayachikitsha, I.P.G.T. simple starch grains, stone cells, pitted vessels. Qualitative analysis and R.A., Gujarat of granules shows that, loss on drying was 5.803 % w/w , ash value H Ayurved University, 1.5248% w/w, P 3.5.Preliminary phyto-chemical screening shows Jamnagar -361008 the presence of Tannin ,Flavonoid ,Steroids, etc. Thin layer Gujarat, India chromatography result show 5 and 4 spots respectively, at 254 nm Mobile no- 9601575811 and 366 nm. -

Scolytus Intricatus (Ratzeburg) Coleoptera: Curculionidae European Oak Bark Beetle
Scolytus intricatus (Ratzeburg) Coleoptera: Curculionidae European Oak Bark Beetle CAPS-Approved Survey Host(s) Method Major/Primary hosts Visual Castanea sativa (Sweet chestnut), Corylus colurna, Quercus spp. (Oak), Quercus coccifera (Kermes oak), Quercus dalechampii (Dalechamp’s oak), Quercus ilex (Holly oak), Quercus petraea (Durmast oak), Quercus robur (Common oak) Other hosts Aesculus spp. (Buckeye), Aesculus hippocastanum (Horse chestnut), Alnus glutinosa (European alder), Betula spp. (Birch), Betula celtiberica (Iberian white birch), Betula pendula (Common silver birch), Betula pubescens (Downy birch), Betula verrucosa (European white birch), Carpinus spp. (Hornbeam), Carpinus betulus (European hornbeam), Castanea spp. (Chestnut), Castanea vesca (Sweet chestnut), Corylus spp. (Hazelnut), Fagus spp. (Beech), Fagus moesiaca, Fagus orientalis (Oriental beech), Fagus sylvatica (Common beech), Ostrya spp. (Hop-hornbeam), Ostrya carpinifolia (Hop-hornbeam), Parrotia persica (Persian parrotia), Populus spp. (Poplars), Populus alba (White poplar), Populus tremula (European aspen), Quercus canariensis (Algerian oak), Quercus castaneaefolia (Chestnut-leaf oak), Quercus cerris (European turkey oak), Quercus frainetto (Italian oak), Quercus hartwissiana, 1 Quercus lusitanica (Lusitanian oak), Quercus polycarpa, Quercus prinus var. tomentosa, Quercus pubescens (Downy oak), Quercus pyrenaica (Pyrenean oak), Quercus rubra (Northern red oak), Quercus virgiliana (Italian oak), Salix spp. (Willow), Sorbus spp. (Mountain Ash), Tilia cordata (Small-leaf lime), Ulmus spp. (Elms), Ulmus carpinifolia (English elm), Ulmus laevis (European white elm), Zelkova carpinifolia (Caucasian zelkova) (Davis et al., 2006; Kimoto and Duthie-Holt, 2006; CABI, 2008) Reason for Inclusion in Manual Scolytus intricatus was added to the manual in 2010. Scolytus intricatus is a target pest on the FY2011 AHP Prioritized Pest List. Pest Description Eggs: Eggs are a pearly white color and oval 1 with a length of 1 mm (< /16 in) (Haack, 2001). -

Download/Standard/270/Pm10-008-1-En.Pdf EPPO (2013) Standard PM 9/14 (1) Agrilus Planipennis: Procedures for Official Control
EUROPEAN AND MEDITERRANEAN PLANT PROTECTION ORGANIZATION ORGANISATION EUROPEENNE ET MEDITERRANEENNE POUR LA PROTECTION DES PLANTES 19-25044 Pest Risk Analysis for Agrilus bilineatus (Coleoptera: Buprestidae), two-lined chestnut borer E. Jendek – EPPO Global Database (EPPO Code: AGRLBL) - Adult of Agrilus bilineatus, Ottawa (USA) September 2019 EPPO 21 Boulevard Richard Lenoir 75011 Paris www.eppo.int [email protected] The risk assessment follows EPPO standard PM 5/5(1) Decision-Support Scheme for an Express Pest Risk Analysis (available at http://archives.eppo.int/EPPOStandards/pra.htm), as recommended by the Panel on Phytosanitary Measures. Pest risk management (detailed in ANNEX 1) was conducted according to the EPPO Decision-support scheme for quarantine pests PM 5/3(5). The risk assessment uses the terminology defined in ISPM 5 Glossary of Phytosanitary Terms (available at https://www.ippc.int/index.php). Cite this document as: EPPO (2019) Pest risk analysis for Agrilus bilineatus. EPPO, Paris. Available at https://gd.eppo.int/taxon/AGRLBL/documents Based on this PRA, Agrilus bilineatus was added to the EPPO A2 Lists of pests recommended for regulation as quarantine pests in 2019. Measures for Castanea and Quercus plants for planting, and wood are recommended. Pest Risk Analysis for Agrilus bilineatus (Coleoptera: Buprestidae), two-lined chestnut borer PRA area: EPPO region Prepared by: Expert Working Group (EWG) on Agrilus fleischeri and A. bilineatus Date: 3-7 December 2018. Further reviewed and amended by EPPO core members and Panel on Phytosanitary Measures (see below). Comments by the Panel on Quarantine Pest for Forestry have also been considered. Composition of the Expert Working Group (EWG) BARANCHIKOV Yuri (Mr) V.