(PMMA) Metabolite Pattern and Influence of CYP2D6 Genetics In
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(Mdma) in Non Conventional Matrices and Its Applications in Clinical Toxicology
Doctoral Thesis Doctorat Farmacología Departament de Farmacología, de Terapéutica i de Toxicologia DISTRIBUTION OF 3,4-METHYLENEDIOXYMETHAMPHETAMINE (MDMA) IN NON CONVENTIONAL MATRICES AND ITS APPLICATIONS IN CLINICAL TOXICOLOGY SIMONA PICHINI p Doctoral Thesis Doctorat Farmacología Departament de Farmacología, de Terapéutica i de Toxicologia DISTRIBUTION OF 3,4-METHYLENEDIOXYMETHAMPHETAMINE (MDMA) IN NON CONVENTIONAL MATRICES AND ITS APPLICATIONS IN CLINICAL TOXICOLOGY Doctoral thesis submitted by Simona Pichini as a partial fulfillment of the requirements for the degree of Doctor by the Universitat Autònoma de Barcelona. The studies included in this thesis have been realized under the direction of Dr. Rafael de la Torre Fornell and Dr. Magí Farré Albaladejo at the Pharmacology Unit of the Institut Municipal d'Investigació Mèdica (IMIM), Barcelona, Spain; and at the Drug Research and Control Department of the Istituto Superiore di Sanità, Roma, Italy. Doctorate Program of the Universitat Autònoma de Barcelona. Signature of Thesis Director Signature of Thesis Director (Dr. Magí Farré Albaladejo) (Dr. Rafael de la Torre Fornell) Signature of Doctorand (Simona Pichini) Yo soy yo y aquéllos a quienes amo Jorge Bucay To my friends, treasure of my life Acknowledgements Acknowledgements A number of people contributed to high extent to achieve the objectives of this Doctoral Thesis prepared between the two cities of Rome and Barcelona. This means that there are many people I’d the opportunity to meet, to work with, to share wonderful and terrible moments, and that I’d like to thank in these pages. First of all, to Dr. Piergiorgio Zuccaro, my “creator”, who always believed in me, supported my job and hardly fought to give me all the possible opportunities to grow up as an investigator; To Dr. -

(19) United States (12) Patent Application Publication (10) Pub
US 20130289061A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2013/0289061 A1 Bhide et al. (43) Pub. Date: Oct. 31, 2013 (54) METHODS AND COMPOSITIONS TO Publication Classi?cation PREVENT ADDICTION (51) Int. Cl. (71) Applicant: The General Hospital Corporation, A61K 31/485 (2006-01) Boston’ MA (Us) A61K 31/4458 (2006.01) (52) U.S. Cl. (72) Inventors: Pradeep G. Bhide; Peabody, MA (US); CPC """"" " A61K31/485 (201301); ‘4161223011? Jmm‘“ Zhu’ Ansm’ MA. (Us); USPC ......... .. 514/282; 514/317; 514/654; 514/618; Thomas J. Spencer; Carhsle; MA (US); 514/279 Joseph Biederman; Brookline; MA (Us) (57) ABSTRACT Disclosed herein is a method of reducing or preventing the development of aversion to a CNS stimulant in a subject (21) App1_ NO_; 13/924,815 comprising; administering a therapeutic amount of the neu rological stimulant and administering an antagonist of the kappa opioid receptor; to thereby reduce or prevent the devel - . opment of aversion to the CNS stimulant in the subject. Also (22) Flled' Jun‘ 24’ 2013 disclosed is a method of reducing or preventing the develop ment of addiction to a CNS stimulant in a subj ect; comprising; _ _ administering the CNS stimulant and administering a mu Related U‘s‘ Apphcatlon Data opioid receptor antagonist to thereby reduce or prevent the (63) Continuation of application NO 13/389,959, ?led on development of addiction to the CNS stimulant in the subject. Apt 27’ 2012’ ?led as application NO_ PCT/US2010/ Also disclosed are pharmaceutical compositions comprising 045486 on Aug' 13 2010' a central nervous system stimulant and an opioid receptor ’ antagonist. -

Acquired Synaesthesia Following 2C-B Use
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Greenwich Academic Literature Archive Acquired synaesthesia following 2C-B use • Authors • Authors and affiliations • Steliana Yanakieva (Department of Psychology, Goldsmiths, University of London) • David P. Luke (Department of Psychology, Social Work and Counselling, University of Greenwich) • Ashok Jansari (Department of Psychology, Goldsmiths, University of London) • Devin B. Terhune (Department of Psychology, Goldsmiths, University of London) Letter to Editor This study was previously presented at Bridging senses: New developments in synesthesia (Royal Society, London, UK, 22–23 October 2018). Psychedelic drugs reliably trigger experiences that closely resemble synaesthesia (Luke and Terhune 2013), a condition in which inducer stimuli will reliably and automatically elicit atypical concurrent experiences (Ward 2013). These transient episodes are considered controversial because they do not meet behavioural diagnostic criteria for developmental synaesthesia (Terhune et al. 2016). However, if these behavioural markers are attributable to the consolidation of synaesthetic associations over time (Terhune et al. 2016), they should be observed in cases of acquired synaesthesia. Here we report a case of drug-induced acquired synaesthesia (LW) that meets standard diagnostic criteria for developmental synaesthesia. LW is a 29-year-old male who reports continuously experiencing multiple forms of synaesthesia for over 7 years since ingesting approximately 70–150 mg of 2,5-dimethoxy-4- bromophenethylamine (2C-B) (Papaseit et al. 2018), which greatly exceeds the normal dosage (12–24 mg) (Shulgin and Shulgin 1990) (see Supplementary Materials). LW was contrasted against 10 non-synaesthete healthy controls, all of whom provided informed written consent. -

Drugs and Medical Devices Group
The Deputy Administrator of the Drug Enforcement Administration (DEA) issued a notice of intent temporarily placing the substance 2,5-dimethoxy-4-(n)- propylthiophenethylamine (2C-T-7), including its optical isomers, salts, and salts of isomers into Schedule I of the Federal Controlled Substances Act (CSA). 2C-T-7 is structurally related to the Schedule I substance 4-bromo-2,5-dimethoxyphenethylamine (2C-B), and it has those structural features of phenethylamines which are necessary for stimulant and/or hallucinogenic activity. There is no approved therapeutic use of 2C-T-7 in the United States, and the safety of this substance has never been demonstrated. This action was based on the following: (1) 2,5-dimethoxy-4-(n)-propylthiophenethylamine is structurally and pharmacologically related to other Schedule I hallucinogens; (2) 2,5-dimethoxy-4-(n)-propylthiophenethylamine has no accepted therapeutic use in the United States and is not safe for use under medical supervision; and, (3) 2,5-dimethoxy-4-(n)-propylthiophenethylamine has a high potential for abuse, similar to other Schedule I phenethylamines. Pursuant to Section 481.034(g), as amended by the 75th legislature, of the Texas Controlled Substances Act, Chapter 481, Health and Safety Code, at least thirty-one days have expired since notice of the above referenced action was published in the Federal Register, and in my capacity as Commissioner of the Texas Department of Health, I do hereby order that the substance 2,5-dimethoxy-4-(n)-propylthiophenethylamine (2C-T-7), its optical isomers, salts, and salts of isomers be added to Schedule I of the Texas Controlled Substances Act. -

Poisons Act.Fm
LAWS OF BRUNEI CHAPTER 114 POISONS Enactment No. 13 of 1956 Amended by S 103/1958 Enactment No. 6 of 1967 S 101/1979 1984 Edition, Chapter 114 Amended by S 16/1996 S 28/2001 GN 273/2002 REVISED EDITION 2015 B.L.R.O. 1/2015 LAWS OF BRUNEI Poisons CAP. 114 1 LAWS OF BRUNEI REVISED EDITION 2015 CHAPTER 114 POISONS ARRANGEMENT OF SECTIONS Section 1. Citation. 2. Interpretation. 3. Description of poisons. 4. Licensing Officers. 5. General prohibition with respect to importation and sale of poisons. 6. Prohibitions and provisions relating to sale of poisons. 7. Exemptions in respect of medicines. 8. Exemptions in respect of sale. 9. Possession of poisons. 10. Issue of licences. 11. Different kinds of licence. 12. Licences to be numbered and registered. 13. Annual list to be published. 14. Forms of licence. 15. Search and search warrants. 16. Powers of exemption. 17. Penalties. B.L.R.O. 1/2015 LAWS OF BRUNEI 2 CAP. 114 Poisons 18. Jurisdiction. 19. Prosecutions. 20. Prohibition of sale to persons under 18. 21. Rules. SCHEDULE — POISONS LIST ____________________________ LAWS OF BRUNEI Poisons CAP. 114 3 POISONS ACT An Act to regulate the importation, possession, manufacture, compounding, storage, transport and sale of poisons Commencement: 1st January 1983 [S 61/1957] Citation. 1. This Act may be cited as the Poisons Act. Interpretation. 2. In this Act, unless the context otherwise requires — “dentist” means a dentist licensed under the Medical Practitioners and Dentists Act (Chapter 112) and includes a Government dentist; “export”, in relation -

The Effects of Low Dose Lysergic Acid Diethylamide Administration in a Rodent Model of Delay Discounting
Western Michigan University ScholarWorks at WMU Dissertations Graduate College 6-2020 The Effects of Low Dose Lysergic Acid Diethylamide Administration in a Rodent Model of Delay Discounting Robert J. Kohler Western Michigan University, [email protected] Follow this and additional works at: https://scholarworks.wmich.edu/dissertations Part of the Biological Psychology Commons Recommended Citation Kohler, Robert J., "The Effects of Low Dose Lysergic Acid Diethylamide Administration in a Rodent Model of Delay Discounting" (2020). Dissertations. 3565. https://scholarworks.wmich.edu/dissertations/3565 This Dissertation-Open Access is brought to you for free and open access by the Graduate College at ScholarWorks at WMU. It has been accepted for inclusion in Dissertations by an authorized administrator of ScholarWorks at WMU. For more information, please contact [email protected]. THE EFFECTS OF LOW DOSE LYSERGIC ACID DIETHYLAMIDE ADMINISTRATION IN A RODENT MODEL OF DELAY DISCOUNTING by Robert J. Kohler A dissertation submitted to the Graduate College In partial fulfillment of the requirements for the degree of Doctor of Philosophy Psychology Western Michigan University June 2020 Doctoral Committee: Lisa Baker, Ph.D., Chair Anthony DeFulio, Ph.D. Cynthia Pietras, Ph.D. John Spitsbergen, Ph.D. Copyright by Robert J. Kohler 2020 THE EFFECTS OF LOW DOSE LYSERGIC ACID DIETHYLAMIDE ADMINISTRATION IN A RODENT MODEL OF DELAY DISCOUNTING Robert J. Kohler, Ph.D. Western Michigan University, 2020 The resurgence of Lysergic Acid Diethylamide (LSD) as a therapeutic tool requires a revival in research, both basic and clinical, to bridge gaps in knowledge left from a previous generation of work. Currently, no study has been published with the intent of establishing optimal microdose concentrations of LSD in an animal model. -

CAS Number Index
2334 CAS Number Index CAS # Page Name CAS # Page Name CAS # Page Name 50-00-0 905 Formaldehyde 56-81-5 967 Glycerol 61-90-5 1135 Leucine 50-02-2 596 Dexamethasone 56-85-9 963 Glutamine 62-44-2 1640 Phenacetin 50-06-6 1654 Phenobarbital 57-00-1 514 Creatine 62-46-4 1166 α-Lipoic acid 50-11-3 1288 Metharbital 57-22-7 2229 Vincristine 62-53-3 131 Aniline 50-12-4 1245 Mephenytoin 57-24-9 1950 Strychnine 62-73-7 626 Dichlorvos 50-23-7 1017 Hydrocortisone 57-27-2 1428 Morphine 63-05-8 127 Androstenedione 50-24-8 1739 Prednisolone 57-41-0 1672 Phenytoin 63-25-2 335 Carbaryl 50-29-3 569 DDT 57-42-1 1239 Meperidine 63-75-2 142 Arecoline 50-33-9 1666 Phenylbutazone 57-43-2 108 Amobarbital 64-04-0 1648 Phenethylamine 50-34-0 1770 Propantheline bromide 57-44-3 191 Barbital 64-13-1 1308 p-Methoxyamphetamine 50-35-1 2054 Thalidomide 57-47-6 1683 Physostigmine 64-17-5 784 Ethanol 50-36-2 497 Cocaine 57-53-4 1249 Meprobamate 64-18-6 909 Formic acid 50-37-3 1197 Lysergic acid diethylamide 57-55-6 1782 Propylene glycol 64-77-7 2104 Tolbutamide 50-44-2 1253 6-Mercaptopurine 57-66-9 1751 Probenecid 64-86-8 506 Colchicine 50-47-5 589 Desipramine 57-74-9 398 Chlordane 65-23-6 1802 Pyridoxine 50-48-6 103 Amitriptyline 57-92-1 1947 Streptomycin 65-29-2 931 Gallamine 50-49-7 1053 Imipramine 57-94-3 2179 Tubocurarine chloride 65-45-2 1888 Salicylamide 50-52-2 2071 Thioridazine 57-96-5 1966 Sulfinpyrazone 65-49-6 98 p-Aminosalicylic acid 50-53-3 426 Chlorpromazine 58-00-4 138 Apomorphine 66-76-2 632 Dicumarol 50-55-5 1841 Reserpine 58-05-9 1136 Leucovorin 66-79-5 -
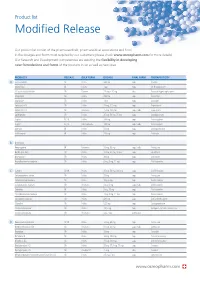
Modified Release
Product list Modified Release Our product list consist of the pharmaceuticals, pharmaceutical associations and food in the dosages and forms most required by our customers (please check www.osmopharm.com for more details). Our Research and Development competencies are assuring the flexibility in developing new formulations and forms of the products in list as well as new ones. PRODUCTS RELEASE BULK FORM DOSAGE FINAL FORM THERAPEUTICITY A Acetazolamide SR Pellets 500 mg caps Diuretic Alfacalcidol IR Pellets 1 μg caps Vit D supplement Alfuzosin Hydrochloride SR Powder 2.5 mg – 10 mg tabs Prostatic Hypertrophy agent Allopurinol SR Pellets 300 mg caps Antiurolytic Alprazolam SR Pellets 1 mg caps Anxiolytic Ambroxol HCI SR Pellets 75 mg, 120 mg caps Expectorant Ambroxol HCI SR Resinates 75 mg, 120 mg caps / tabs Expectorant Amitriptyline SR Pellets 25 mg, 50 mg, 75 mg caps Antidepressant Aspirin EC, IR Pellets 100 mg caps Anticoagulant Aspirin EC, IR Microcapsules 100 mg caps / tabs Anticoagulant Atenolol IR Pellets 50 mg caps Antihypertensive Azithromycin IR Pellets 250 mg caps Antibiotic B B-complex Benproperine IR Resinates 25 mg, 50 mg caps / tabs Antitussive Betahistine 2HCI SR Pellets 12 mg, 24 mg, 48 mg caps Vasodilator Bromopride* SR Pellets 20 mg caps Antiemetic Brompheniramine maleate SR Pellets 6 mg, 8 mg, 12 mg caps Antihistaminic C Caffeine SR, IR Pellets 25 mg, 50 mg, 300 mg caps CNS Stimulant Carbetapentane citrate SR Pellets 75 mg caps Antitussive Carbinoxamine maleate SR Pellets 4mg, 6 mg caps Antihistaminic Carbinoxamine -

West Virginia Secretary of State Administrative Law Division
WEST VIRGINIA Do Not Mark In This Box SECRETARY OF STATE NATA LIE E. TENNANT ADMINISTRATIVE LAW DIVISION Form #2 ·---------------------------'-------~----- NOTICE OF A COMMENT PERIOD ON A PROPOSED RULE AGENCY: West Virginia Racing Commission TITLE NUMBER: 178 RULE TYPE: _Le_g_isl_at_iv_e--------- CITE AUTHORITY: WV Code 19-23-2(al. 19-23-3071. 19-23-6. 19-23-8, 19-23-9. 19-23-13, 19-23-13b. 19-23-15 AMENDMENT TO AN EXISTING RULE: YES- NO- IF YES, SERIES NUMBER OF RULE BEING AMENDED: ----- TITLE OF RULE BEING AMENDED:Th_or_ou...:g_hb_re_d_Ra_c_in.::.g ________________ IF NO, SERIES NUMBER OF RULE BEING PROPOSED:----- TITLE OF RULE BEING PROPOSED:-------------------- IN LIEU OF A PUBLIC HEARING, A COMMENT PERIOD HAS BEEN ESTABLISHED DURING WHICH ANY INTERESTED PERSON MAY SEND COMMENTS CONCERNING THESE PROPOSED RULES. THIS COMMENT PERIOD WILL END ON July 19 • 2013 AT 5:00p.m. ONLY WRITTEN COMMENTS WILL BE ACCEPTED AND ARE TO BE MAILED TO THE FOLLOWING ADDRESS: West Virginia Racing Commission 900 Pennsylvania A venue, Suite 533 THE ISSUES TO BE HEARD SHALL BE LIMITED TO THIS PROPOSED RULE. Charleston, West Virginia 25302 tegislathte Rule Making JUN 1 9 2013 Secretary Review Committee BRIEF SUMMARY OF PROPOSAL THOROUGHBRED RACING 178 CSR I The proposed amendments to the rule are intended to update, clarify and address problems with the rule so that the West Virginia Racing Commission can effectively regulate thoroughbred racing in this State. In 2011, the Commission completely re-wrote, repealed and replaced the existing Thoroughbred Racing Rule that was effective April6, 2007. In going through the re-write process in 20 II, the Commission recognized that a more frequent examination of its rule is necessary to keep abreast of industry standards and to address issues with the rule that arise from time to time. -

Lysergic Acid Diethylamide (LSD) Promotes Social Behavior Through Mtorc1 in the Excitatory Neurotransmission
Lysergic acid diethylamide (LSD) promotes social behavior through mTORC1 in the excitatory neurotransmission Danilo De Gregorioa,b,1, Jelena Popicb,2,3, Justine P. Ennsa,3, Antonio Inserraa,3, Agnieszka Skaleckab, Athanasios Markopoulosa, Luca Posaa, Martha Lopez-Canula, He Qianzia, Christopher K. Laffertyc, Jonathan P. Brittc, Stefano Comaia,d, Argel Aguilar-Vallese, Nahum Sonenbergb,1,4, and Gabriella Gobbia,f,1,4 aNeurobiological Psychiatry Unit, Department of Psychiatry, McGill University, Montreal, QC, Canada, H3A 1A1; bDepartment of Biochemistry, McGill University, Montreal, QC, Canada, H3A 1A3; cDepartment of Psychology, McGill University, Montreal, QC, Canada, H3A 1B1; dDivision of Neuroscience, Vita Salute San Raffaele University, 20132 Milan, Italy; eDepartment of Neuroscience, Carleton University, Ottawa, ON, Canada, K1S 5B6; and fMcGill University Health Center, Montreal, QC, Canada, H3A 1A1 Contributed by Nahum Sonenberg, November 10, 2020 (sent for review October 5, 2020; reviewed by Marc G. Caron and Mark Geyer) Clinical studies have reported that the psychedelic lysergic acid receptor (6), but also displays affinity for the 5-HT1A receptor diethylamide (LSD) enhances empathy and social behavior (SB) in (7–9). Several studies have demonstrated that LSD modulates humans, but its mechanism of action remains elusive. Using a glutamatergic neurotransmission and, indirectly, the α-amino- multidisciplinary approach including in vivo electrophysiology, 3-hydroxy-5-methyl-4-isoxazole propionate (AMPA) receptors. optogenetics, behavioral paradigms, and molecular biology, the Indeed, in vitro studies have shown that LSD increased the ex- effects of LSD on SB and glutamatergic neurotransmission in the citatory response of interneurons in the piriform cortex following medial prefrontal cortex (mPFC) were studied in male mice. -

Pdf 36.06 Kb
PROJECT REVIEW “Synthesis and Characterisation of Metabolites for the Integration in a Comprehensive Screening Procedure utilizing LC-MS/MS” W. Schänzer, M. Parr (German Sport University, Cologne, Germany) In the fight against doping the laboratories are confronted with an increasing number of substances to screen on. Therefore new methods have to be implemented by the laboratories. To keep the costs for doping control analysis acceptable, to ensure rapid reporting times and to lower the amount of urine needed to screen for all substances, a comprehensive screening for different classes of substances is desirable. Following the 2005 application WADA has granted a pilot project to check for the applicability of direct LC-MS/MS measurement of sulfoconjugates of heavy volatile stimulants. As most of the beta-2 agonists and heavy volatilestimulants are conjugated to sulfuric acid in humans an extension of the method to other compounds is desirable. As reference substances of the conjugates are barely available, during the pilot project the sulfoconjugates of p-Hydroxyamphetamine, p-Hydroxymetamphetamine (Pholedrine), p- Hydroxyephedrine, p-Hydroxynorephedrine, Etilefrine and Etamivan were synthesized by coupling the aglycons to sulfuric acid by reaction with sulfur trioxide pyridine complex. The objective of the continuation is to extend the combined screening procedure for diuretics and heavy volatile stimulants developed in the pilot project to other compounds. Thus, studies on the metabolism have to be reviewed and relevant metabolites have to be synthesised. The structures of all relevant products will be confirmed by nuclear magnetic resonance. For the integration in a comprehensive screening procedure the analytes will be characterised by means of LC-MS/MS. -

4-Bromo-2,5-Dimethoxyphenethylamine (Street Names: 2C-B, Nexus, 2’S, Toonies, Bromo, Spectrum, Venus)
Drug Enforcement Administration Diversion Control Division Drug & Chemical Evaluation Section 4-Bromo-2,5-Dimethoxyphenethylamine (Street Names: 2C-B, Nexus, 2’s, Toonies, Bromo, Spectrum, Venus) February 2020 User Population: Introduction: 2C-B is used by the same population as those using 4-Bromo-2,5-dimethoxyphenethylamine (2C-B, 4- “Ecstasy” and other club drugs; high school and college bromo-2,5-DMPEA) is a synthetic schedule I hallucinogen. students, and other young adults who frequent “rave” or It is abused for its hallucinogenic effects primarily as a club “techno” parties. drug in the rave culture and “circuit” party scene. Illicit Distribution: Licit Uses: 2C-B is distributed as tablets, capsules, or in powder 2C-B has no approved medical uses in the United form. Usually sold as MDMA, a single dosage unit of 2C-B States. typically sells for $10 to $30 per tablet. The illicit source of 2C-B currently available on the street has not been Chemistry and Pharmacology: identified by DEA. Prior to its control, DEA seized both 4-Bromo-2,5-dimethoxyphenethylamine is closely clandestine laboratories and illicit “repacking shops.” As related to the phenylisopropylamine hallucinogen 1-(4- the name implies, these shops would repackage and bromo-2, 5-dimethoxyphenyl)-2-aminopropane (DOB) and reformulate the doses of the tablets prior to illicit sales. is referred to as alpha-desmethyl DOB. 2C-B produces According to the System to Retrieve Information from effects similar to 2,5-dimethoxy-4-methylamphetamine Drug Evidence (STRIDE) data, the first recorded (DOM) and DOB. 2C-B displays high affinity for central submission by law enforcement to DEA forensic serotonin receptors.