(Dimethylamino)Propylamine
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Safety Assessment of Alkyl Betaines As Used in Cosmetics
Safety Assessment of Alkyl Betaines as Used in Cosmetics Status: Draft Tentative Report for Panel Review Release Date: November 15, 2013 Panel Meeting Date: December 9-10, 2013 The 2013 Cosmetic Ingredient Review Expert Panel members are: Chairman, Wilma F. Bergfeld, M.D., F.A.C.P.; Donald V. Belsito, M.D.; Curtis D. Klaassen, Ph.D.; Daniel C. Liebler, Ph.D.; Ronald A. Hill, Ph.D. James G. Marks, Jr., M.D.; Ronald C. Shank, Ph.D.; Thomas J. Slaga, Ph.D.; and Paul W. Snyder, D.V.M., Ph.D. The CIR Director is Lillian J. Gill, DPA. This safety assessment was prepared by Christina L. Burnett, Scientific Analyst/Writer, and Bart Heldreth, Ph.D., Chemist CIR. © Cosmetic Ingredient Review 1101 17th Street, NW, Suite 412 Washington, DC 20036-4702 ph 202.331.0651 fax 202.331.0088 [email protected] Distributed for Comment Only -- Do Not Cite or Quote Commitment & Credibility since 1976 Memorandum To: CIR Expert Panel Members and Liaisons From: Christina L. Burnett Scientific Writer/Analyst Date: November 15, 2013 Subject: Draft Tentative Report on Alkyl Betaines At the September 2013 CIR Expert Panel Meeting, the Panel issued an insufficient data announcement on the safety assessment of alkyl betaine ingredients. The data needs included: (1) method of manufacturing; and (2) impurities. Since the announcement, we have received method of manufacturing and impurities data from a supplier of coco- betaine. This data along with basic composition data on a betaine analog from the European Chemicals Agency (ECHA) database and basic method of manufacturing data on food-grade betaine have been incorporated in the report. -

Cocamidopropyl Betaine
COCAMIDOPROPYL BETAINE Your patch test result indicates that you have a contact allergy to cocamidopropyl betaine. This contact allergy may cause your skin to react when it is exposed to this substance although it may take several days for the symptoms to appear. Typical symptoms include redness, swelling, itching and fluid-filled blisters. Where is cocamidopropyl betaine found? Cocamidopropyl betaine is used in personal care products like shampoos, hand soaps, and toothpastes, and in cosmetics as an emulsifying agent and thickener. It is also used in conditioners to reduce static cling. How can you avoid contact with cocamidopropyl betaine? Avoid products that list any of the following names in the ingredients: • 1-Propanaminium, N-(carboxymethyl)- N,N-dimethyl-3-((1-oxococonut)amino)-, hydroxide, inner salt • N-(2-Aminoethyl)-N-(2-(2- carboxyethoxy)ethyl) beta-alanine, norcoco acyl derivs., disodium salts • N-(Carboxymethyl)-N,N-dimethyl-3-((1- oxococonut)amino)-1-propanam- inium • Cocamidopropyl betaine • Cocamidopropyl dimethyl glycine • CAS RN: 61789-40-0 What are some products that may contain cocamidopropyl betaine? Anti-fungal: Shampoo/Conditioner: • Mycocide NS • Alberto VO5 Extra Body Shampoo • Alberto VO5 Herbal Escapes Moisturizing Shampoo Cosmetics: • Alberto VO5 Moisture Milks Nourishing Shampoo • Gillette Multi-Glide Shave Gel B,CPB • Alberto VO5 Normal Shampoo Hair Coloring Kits: • Aveeno Baby Wash & Shampoo • Clairol Natural Instincts Haircolor, Level 2, Hazelnut-20 • Charles Worthington Big Hair Full Volume Shampoo • -

Cocamidopropyl Betaine (CAPB)
Final Report to DWI September 2020 Personal Care Products and Domestic Cleaning Products – Toxicological Assessment of Prioritised List of Chemicals (Ref: DWI 70/2/331) FINAL REPORT Report to Defra/DWI IEH Consulting Ltd.|September 2020 Final Report to DWI September 2020 PCPs and DCPs – toxicological assessment of prioritised list of chemicals A Report to DWI by IEH Consulting Ltd. Disclaimer The views expressed in this report are those of the authors alone, representing their expert opinion based on the review of the available information and their understanding drawn from publications identified from targeted literature searches. Prepared by: Ruth Bevan, Sarah Bull Reviewed by: Camilla Alexander-White, Paul Rumsby Edited by: Paul Harrison September 2020 Final Report to DWI September 2020 Contents Contents Abbreviations ............................................................................................................................. 8 Executive Summary .................................................................................................................... 9 1.0 Introduction ....................................................................................................................... 11 1.1 Project Background ............................................................................................................ 11 1.2 Personal Care Products ...................................................................................................... 11 1.3 Domestic Cleaning Products ............................................................................................. -

Determination of SLES in Personal Care Products by Colloid Titration with Light Reflection Measurements
molecules Article Determination of SLES in Personal Care Products by Colloid Titration with Light Reflection Measurements Dorota Ziółkowska 1,* , Iryna Syrotynska 2 , Alexander Shyichuk 1 and Jan Lamkiewicz 1 1 Faculty of Chemical Technology and Engineering, UTP University of Science and Technology, Seminaryjna 3, 85-326 Bydgoszcz, Poland; [email protected] (A.S.); [email protected] (J.L.) 2 Biochemistry Department, Ivano-Frankivsk National Medical University, Halyts’ka 2, 76000 Ivano-Frankivs’k, Ukraine; [email protected] * Correspondence: [email protected] Abstract: The method of colloid titration with poly(diallyldimethylammonium) chloride has been improved to detect the endpoint with an off-vessel light reflectance sensor. The digital color sensor used measures light reflectance by means of light guides, with no immersion into the reaction solution. In such a method, the optical signal is free of disturbances caused by sticky flocs in the solution. The improved automatic titration set was applied for the determination of sodium laureth sulfate (SLES) in industrial batches and commercial personal care products. The sample color and opacity do not disturb the SLES quantification. When the SLES content lies in the range from 5% to 9%, the optimal sample weight is from 6 g to 3 g. Keywords: quantitation of surfactants; turbidity; polyDADMAC; PDADMAC; PDDA; cationic polymer Citation: Ziółkowska, D.; Syrotynska, I.; Shyichuk, A.; Lamkiewicz, J. Determination of SLES in Personal Care Products by Colloid 1. Introduction Titration with Light Reflection Sodium laureth sulfate (SLES) is a common primary surfactant in skin and hair Measurements. Molecules 2021, 26, care products [1–3]. Therefore, the determination of SLES concentration in personal care 2716. -
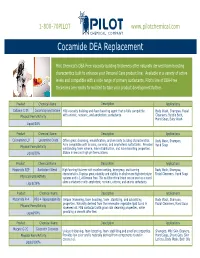
Cocamide DEA Replacement
1-800-70PILOT www.pilotchemical.com Cocamide DEA Replacement Pilot Chemical's DEA-Free viscosity building thickeners offer naturally derived foam boosting characteritics built to enhance your Personal Care product line. Available in a variety of active levels and compatible with a wide range of primary surfactants, Pilot's line of DEA-Free thickeners are readily formulated to take your product development further. Product Chemical Name Description Applications Caltaine C-35 Cocamidopropyl Betaine Mild viscosity building and foam boosting agent that is fully compatible Body Wash, Shampoo, Facial Physical Form/Activity with anionic, nonionic, and amphoteric surfactants. Cleansers, Bubble Bath, Hand Soap, Baby Wash Liquid/30% Product Chemical Name Description Applications Caloxamine LO Lauramine Oxide Offers great cleansing, emulsification, and viscosity building characteristics. Body Wash, Shampoo, Fully compatible with anionic, nonionic, and amphoteric surfactants. Provides Hand Soap Physical Form/Activity outstanding foam volume, foam stabiliaztion, and foam boosting properties. Liquid/30% Stable in low and high pH formulations. Product Chemical Name Description Applications Masamide REP Surfactant Blend High foaming thickener with excellent wetting, detergency, and foaming Body Wash, Shampoo, characteristics. Displays great solubility and stability in alkaline and high electrolyte Facial Cleansers, Hand Soap Physical Form/Activity systems and is 1,4-Dioxane Free. This multifunctional blend can be used as a stand Liquid/36% alone surfactant or with amphoteric, nonionic, cationic, and anionic surfactants. Product Chemical Name Description Applications Masamide R-4 PEG-4 Rapeseedamide Unique thickening, foam boosting, foam stabilizing, and solubilizing Body Wash, Shampoo, properties. Naturally derived from the renewable vegetable lipid found in Facial Cleansers, Hand Soap Physical Form/Activity rapeseed oil. -

Alternatives to Cocamide DEA California's Office of Environmental Health Hazard Assessment (OEHHA) Has Listed Cocamide DEA (CAS No
Alternatives to Cocamide DEA California's Office of Environmental Health Hazard Assessment (OEHHA) has listed Cocamide DEA (CAS No. 68603-42-9) on Proposition 65. For those looking to reformulate, Stepan is providing the following performance comparison and alternative surfactant recommendations to NINOL® 40-CO (1:1 Cocamide DEA) and NINOL® 11-CM (2:1 Cocamide DEA) for use in liquid dish detergents, all-purpose cleaners, degreasers, and vehicle care detergents. Stepan has a broad amphoteric product line and the technical expertise in hard surface care to assist our customers with product recommendation and reformulation. The recommended surfactants noted below take into consideration performance, handling, typical lead time, and estimated market price. If you require further assistance, please contact your local Stepan sales representative or Stepan U.S. Technical Service at [email protected] or (800) 745-7837. LIQUID DISH DETERGENTS (LDL) For an LDL economy formulation containing Linear Alkylbenzene Sulfonate (LAS) and Sodium Laureth Sulfate (SLES), the following surfactants showed equivalent or improved foam mileage and equivalent foam volume compared to NINOL® 40-CO on an equal actives basis. Recommendations Mixer Foam: LAS/SLES Economy AMMONYX® LMDO (Lauramidopropylamine Oxide) 160 AMPHOSOL® CG-50 (Cocamidopropyl Betaine) 150 AMMONYX® CDO SPECIAL (Cocamidopropylamine Oxide) 140 Shake Foam: LAS/SLES Economy 130 Soil D 500 120 Shell 400 110 Soil 300 100 90 200 Normalized Normalized Foam Mileage 80 100 Foam Volume (mL) 0 40-CO LMDO CG-50 CDO SPECIAL For an LDL premium formulation containing Sodium Lauryl Sulfate (SLS) and Sodium Laureth Sulfate (SLES), the following surfactants showed equivalent or improved foam mileage and equivalent foam volume compared to NINOL® 40-CO on an equal actives basis. -

Shampoos for Special Considerations
750 West Broadway Suite 905 - Vancouver BC V5Z 1H8 Phone: 604.283.9299 Fax: 604.648.9003 Email: [email protected] Web: www.donovanmedical.com SHAMPOOS FOR SPECIAL CONSIDERATIONS Many common shampoo products can cause irritation of the scalp. This frequently manifests are redness and itchiness when the products are applied. However, not all products actually cause scalp problems at first – and some start out with irritation of the FEEN- face, eyelids, ears and neck FRAGRANCE- FREE SHAMPOOS Scalp sensitivities can rarely be from fragrance sensitivities. These can also cause reactions on the face and neck. A great majority of shampoos contain fragrance. The following are fragrance Free Shampoos: FREE AND CLEAR SHAMPOO • No fragrance • No Parabens • No SLS (sulphate) • no CAPB purified water, lauryl glucoside, coco-glucoside, acrylates copolymer, disodium cocoyl glutamate, sodium cocoyl glycinate, glycerin, sucrose cocoate, panthenol, pentylene glycol, 1,2-hexanediol, sodium cocoyl glutamate, disodium EDTA, caprylyl glycol, sodium hydroxide, sodium chloride FRAGRANCE- FREE SHAMPOOS (CONTINUED) DUCRAY PHYSIOPROTECTIVE SENSINOL SHAMPOO • No fragrance • No Parabens • No alcohol • no CAPB • Ingredients o WATER (AQUA), SODIUM LAURETH SULFATE, ZINC COCETH SULFATE, CETEARETH-60 MYRISTYL GLYCOL, LAURETH-9, COCO- GLUCOSIDE, 1,2-HEXANEDIOL, CAPRYLYL GLYCOL, CITRIC ACID, DISODIUM EDTA, HYDROXYPROPYL GUAR HYDROXYPROPYLTRIMONIUM CHLORIDE, SODIUM CHLORIDE, SODIUM HYDROXIDE AFM SAFE CHOICE HEAD AND BODY SHAMPOO • No fragrance • No Parabens -

Qualitative Tier 2 Assessment
Consider It Done www.ehs-support.com.au Qualitative Tier 2 Assessment Cocamidopropyl Betaine In accordance with the Chemical Risk Assessment Framework (CRAF), chemicals assigned a Tier 2 designation require a hazard assessment and qualitative assessment of risk. Consistent with National Industrial Chemicals Notification and Assessment Scheme (NICNAS), the human health hazards for each chemical are characterised by analysing the toxicokinetics (the absorption, distribution, metabolism and excretion of the chemical in humans or laboratory animals), acute toxicity, irritation and corrosivity, repeat dose toxicity, genotoxicity, carcinogenicity, reproductive toxicity, and other health effects. The environmental hazards for each chemical are characterized by analysing the environmental fate properties (such as mobility, persistence, bioavailability and bioaccumulation), acute toxicity and chronic toxicity. In support of the hazard assessment, a risk assessment dossier is prepared for each of the chemicals included in the assessment. The qualitative assessment of risk evaluates exposure to the vendor chemical that may occur during activities that do not intentionally result in a release to the environment, but where a potential release may occur. For this evaluation, these potential releases primarily are focused on the vendor chemical transported to the well pad site or water management facility (WMF), chemicals utilised in drilling fluid systems that may impact groundwater, residual chemicals that may be present in hydraulic flowback and workover fluids and chemicals and chemicals and residues of chemicals that may be present in water undergoing treatment or beneficially re-used. Potentially complete exposure pathways (in that a source, a migration pathway, a mechanism for exposure, and a potential receptor are present) are assessed herein to determine the potential for risk (an incomplete pathway precludes an exposure occurring and an associated potential risk). -

Chemical Names (B) Lauramidopropyl Cocamidopropyl Cocamidopropyl Betaine Betaine Sodium Salts Betaine Inner Salts
SIAM 23, 17-20 October 2006 DE/ICCA SIDS INITIAL ASSESSMENT PROFILE Chemical Category Name Alkylamidopropyl betaines 1-Propanaminium, N- 1-Propanaminium, 3- Amides, coco, > (carboxy-methyl)-N,N- amino- N- 3-(dimethyl- @ dimethyl- N-(carboxymethyl)- amino)propyl , N,N-dimethyl-, CATEGORY MEMBERS: 3->(1-oxododecyl) alkylation products @ N-coco acyl with chloroacetic acid, amino -, inner salts (A) derivatives, inner salts sodium salt (C) Chemical Names (B) Lauramidopropyl Cocamidopropyl Cocamidopropyl betaine betaine sodium salts betaine inner salts CAS Registry Numbers 4292-10-8 61789-40-0 70851-07-9 O + O N N A O STRUCTURAL FORMULAS Cl O O - + O + O R N N R N N Na+ O O C B SUMMARY CONCLUSION OF THE SIAR Category Justification The members of the alkylamidopropyl betaines category are amphoteric surfactants containing a quarternary ammonium ion, a carboxylic structure, and an amide bond. The alkylamidopropyl betaines are referred to as inner salts due to their zwitterionic character. They are all manufactured from oils, usually coconut oil, containing mixtures of C8 to C18 fatty acids and marketed as aqueous solutions (20 - 40 %). Because of the structural and functional similarities and comparable physico-chemical properties of cocamidopropyl betaine inner salts and sodium salts, a similar ecotoxicological and toxicological profile can be expected. Values for physico-chemical endpoints for lauramidopropyl betaine are similar or within the range of values for cocamidopropyl betaines, supported by accepted (Q)SARs even though there may be limitations for surface active substances. Therefore similar ecotoxicological properties were assumed. All available physico- chemical and environmental fate data are similar for lauramidopropyl betaine and cocamidopropyl betaine and hence support this category approach. -

Scientific Literature Review Fatty Acid Amidopropyl Dimethylamines As
Scientific Literature Review Fatty Acid Amidopropyl Dimethylamines as Used in Cosmetics February 13, 2012 All interested persons are provided 60 days from the above date to comment on this Scientific Literature Review and to identify additional published data that should be included or provide unpublished data which can be made public and included. Information may be submitted without identifying the source or the trade name of the cosmetic product containing the ingredient. All unpublished data submitted to CIR will be discussed in open meetings, will be available at the CIR office for review by any interested party and may be cited in a peer-reviewed scientific journal. Please submit data, comments, or requests to the CIR Director, Dr. F. Alan Andersen. The 2012 Cosmetic Ingredient Review Expert Panel members are: Chair, Wilma F. Bergfeld, M.D., F.A.C.P.; Donald V. Belsito, M.D.; Ronald A Hill, Ph.D.; Curtis D. Klaassen, Ph.D.; Daniel C. Liebler, Ph.D.; James G. Marks, Jr., M.D.; Ronald C. Shank, Ph.D.; Thomas J. Slaga, Ph.D.; and Paul W. Snyder, D.V.M., Ph.D. The CIR Director is F. Alan Andersen, Ph.D. This report was prepared by Christina Burnett, Scientific Analyst/Writer, and Bart Heldreth, Ph.D., Chemist CIR. © Cosmetic Ingredient Review 1101 17th Street, NW, Suite 412 Washington, DC 20036-4702 ph 202.331.0651 fax 202.331.0088 [email protected] TABLE OF CONTENTS INTRODUCTION ............................................................................................................................................. 3 CHEMISTRY -

Thickening of Foaming Cosmetic Formulations
THICKENING OF FOAMING COSMETIC FORMULATIONS Geert De Lathauwer, Daisy De Rycke, Annelies Duynslager, Stijn Tanghe, Caroline Oudt EOC Surfactants nv, Belgium Abstract Enhancing the viscosity of a foaming cosmetic formulation has both marketing and technical reasons. A rich appearance will be correlated by end users with high concentration and value for money. Thickening also has an advantage in applying the product: a thin shampoo would run off the hands easily. In formulation development and optimization, a thickened formulation can also allow keeping heterogeneous solutions in a stable equilibrium. This paper gives an overview of the different ways of incorporating viscosity in a surfactant formulation including an explanation of the mechanisms involved. It summarizes the different types of raw materials available to formulators illustrating their advantages and drawbacks. The second part gives an overview of typical frame formulations of commercially available shampoos and shower gels. Finally, this paper presents an up-to-date list of most widely used alternatives to diethanolamine based surfactants whilst showing their performance. The comparative study illustrates the superiority of one of the selected alternatives from point of view of workability and similar viscosity build-up performance in a formulation as Cocamide DEA. Introduction Enhancing the viscosity of a foaming cosmetic formulation has both marketing and technical reasons. A rich appearance will be correlated by end users with high concentration and so gives them the impression that they get value for money. Thickening also has an advantage in applying the product: low viscous liquids are difficult to apply to hair and skin; more viscous products will not run off the hands too easily. -

Your Definitive Solution for Personal Care
Your Definitive Solution for Personal Care We are a distributor and manufacturer of ingredients with an extensive raw material portfolio. Surfactants • Acetamide MEA • Glycereth-7 Caprylate/Caprate • Sodium Coco-Sulfate • Alkylpolyglucoside • Glycereth-7 Cocoate • Sodium Cocoyl Glycinate • Ammonium Laureth Sulfate • Hempseedamidopropyl • Sodium Cocoyl Isethionate • Ammonium Lauryl Sulfate Hydroxysultaine • Sodium Cocoyl Sarcosinate • Babassuamidopropyl Hydroxysultaine • Hydrogenated Cocamidopropyl Betaine • Sodium Decylglucosides • C12-18 Alkyldimethyl Amine N-Oxides • Lauramide DIPA Hydroxypropylsulfonate • Calcium Stearoyl Lactylate • Lauramide MEA • Sodium Hydroxypropylphosphate • Capryl/Capramidopropyl Betaine • Lauramide MIPA Cocoglucoside Crosspolymer • Caprylyl/Capryl Glucoside • Lauramidopropyl Betaine • Sodium Hydroxypropylphosphate • Cetyl Betaine • Lauramidopropyl Hydroxysultaine Decylglucoside Crosspolymer • Cocamide DIPA • Lauramidopropylamine Oxide • Sodium Hydroxypropylphosphate Laurylglucoside Crosspolymer • Cocamide MIPA • Lauramine Oxide • Sodium Laureth Sulfate • Cocamidopropyl Betaine • Laureth-11 Carboxylic Acid • Sodium Laureth Sulfosuccinate • Cocamidopropyl Hydroxysultaine • Laureth-6 Carboxylic Acid • Sodium Laureth-11 Carboxylate • Cocamidopropylamine Oxide • Lauryl Betaine • Sodium Laureth-5 Carboxylate • Cocamine Oxide • Lauryl Glucoside • Sodium Laureth-6 Carboxylate • Coco-Betaine • Lauryl Hydroxysultaine • Sodium Lauriminodipropionate • Coco-Glucoside • Lauryl Lactate • Sodium Lauroamphoacetate • Decyl Glucoside