Thickening of Foaming Cosmetic Formulations
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Safety Assessment of Alkyl Betaines As Used in Cosmetics
Safety Assessment of Alkyl Betaines as Used in Cosmetics Status: Draft Tentative Report for Panel Review Release Date: November 15, 2013 Panel Meeting Date: December 9-10, 2013 The 2013 Cosmetic Ingredient Review Expert Panel members are: Chairman, Wilma F. Bergfeld, M.D., F.A.C.P.; Donald V. Belsito, M.D.; Curtis D. Klaassen, Ph.D.; Daniel C. Liebler, Ph.D.; Ronald A. Hill, Ph.D. James G. Marks, Jr., M.D.; Ronald C. Shank, Ph.D.; Thomas J. Slaga, Ph.D.; and Paul W. Snyder, D.V.M., Ph.D. The CIR Director is Lillian J. Gill, DPA. This safety assessment was prepared by Christina L. Burnett, Scientific Analyst/Writer, and Bart Heldreth, Ph.D., Chemist CIR. © Cosmetic Ingredient Review 1101 17th Street, NW, Suite 412 Washington, DC 20036-4702 ph 202.331.0651 fax 202.331.0088 [email protected] Distributed for Comment Only -- Do Not Cite or Quote Commitment & Credibility since 1976 Memorandum To: CIR Expert Panel Members and Liaisons From: Christina L. Burnett Scientific Writer/Analyst Date: November 15, 2013 Subject: Draft Tentative Report on Alkyl Betaines At the September 2013 CIR Expert Panel Meeting, the Panel issued an insufficient data announcement on the safety assessment of alkyl betaine ingredients. The data needs included: (1) method of manufacturing; and (2) impurities. Since the announcement, we have received method of manufacturing and impurities data from a supplier of coco- betaine. This data along with basic composition data on a betaine analog from the European Chemicals Agency (ECHA) database and basic method of manufacturing data on food-grade betaine have been incorporated in the report. -

Cocamidopropyl Betaine
COCAMIDOPROPYL BETAINE Your patch test result indicates that you have a contact allergy to cocamidopropyl betaine. This contact allergy may cause your skin to react when it is exposed to this substance although it may take several days for the symptoms to appear. Typical symptoms include redness, swelling, itching and fluid-filled blisters. Where is cocamidopropyl betaine found? Cocamidopropyl betaine is used in personal care products like shampoos, hand soaps, and toothpastes, and in cosmetics as an emulsifying agent and thickener. It is also used in conditioners to reduce static cling. How can you avoid contact with cocamidopropyl betaine? Avoid products that list any of the following names in the ingredients: • 1-Propanaminium, N-(carboxymethyl)- N,N-dimethyl-3-((1-oxococonut)amino)-, hydroxide, inner salt • N-(2-Aminoethyl)-N-(2-(2- carboxyethoxy)ethyl) beta-alanine, norcoco acyl derivs., disodium salts • N-(Carboxymethyl)-N,N-dimethyl-3-((1- oxococonut)amino)-1-propanam- inium • Cocamidopropyl betaine • Cocamidopropyl dimethyl glycine • CAS RN: 61789-40-0 What are some products that may contain cocamidopropyl betaine? Anti-fungal: Shampoo/Conditioner: • Mycocide NS • Alberto VO5 Extra Body Shampoo • Alberto VO5 Herbal Escapes Moisturizing Shampoo Cosmetics: • Alberto VO5 Moisture Milks Nourishing Shampoo • Gillette Multi-Glide Shave Gel B,CPB • Alberto VO5 Normal Shampoo Hair Coloring Kits: • Aveeno Baby Wash & Shampoo • Clairol Natural Instincts Haircolor, Level 2, Hazelnut-20 • Charles Worthington Big Hair Full Volume Shampoo • -

(Dimethylamino)Propylamine
3-Dimethylaminopropylamine 109-55-7 OVERVIEW This material was prepared for the National Cancer Institute (NCI) for consideration by the Chemical Selection Working Group (CSWG) by Technical Resources International, Inc. under Contract no. N02-07007. 3-Dimethylaminopropylamine (DMAPA) came to the attention of the National Cancer Institute (NCI) Division of Cancer Biology as the result of a review of chemical industry information suggesting an increased production or use of this chemical. DMAPA appears to have a significant and increasing demand for its use in personal care products. This chemical also has applications in the leather processing, paper, and rubber industries and is an intermediate in the production of fabric softeners, polymers, agrochemicals, flocculating agents, liquid soaps, and dye intermediates. There is a lack of information on the chronic toxicity of DMAPA and other chemicals structurally related to DMAPA. In a SIDS screen, DMAPA produced deaths in high dose animals exposed for 28 days, but this chemical lacked reproductive or developmental effects and produced a negative mutagenicity profile. Workers occupationally exposed to DMAPA at concentrations of 0.55-1.38 mg/m3 experienced impaired respiration that may not have been completely reversible even when concentrations of DMAPA were reduced. Given the lack of chronic toxicity data for DMAPA, its high production volume, and its widespread use, additional toxicity testing appears warranted. NOMINATION OF 3-DIMETHYLAMINOPROPYLAMINE TO THE NTP Based on a review of available -

Personal Care
HAIR CONDITIONERS SURFACTANTSS • Behenamidopropyl Dime- pholine Lactate • Acetamide MEA • Lauramidopropyl Betaine thylamine • Myristyl Trimethyl Ammo- • Acetamide MEA, Lactamide • Lauramidopropyl Hydroxysul- • Cocodimonium Hydroxy- nium Bromide MEA taine propyl Hydrolyzed Rice • Polymethacrylamidoprop- • Alkyl Dimethyl (C12-C18) • Lauramidopropylamine Oxide Protein yltrimonium Chloride Amine Oxide • Lauramine Oxide • Cocodimonium Hydroxy- • Polyquaternium-2 • Alkylpolyglucoside • Laureth-1 Phosphate propyl Hydrolyzed Silk • Polyquaternium-6 • Ammonium Laureth Sulfate • Laureth-11 Carboxylic Acid Protein • Polyquaternium-7 • Ammonium Lauryl Sulfate • Laureth-11, Myreth-11 • Cocodimonium Hydroxy- • Polyquaternium-74 • Ammonium Lauryl Sulfate, • Laureth-3 Phosphate propyl Hydrolyzed Wheat • PPG-3 Caprylyl Ether Ammonium Laureth Sulfate • Laureth-6 Carboxylic Acid Protein • Quaternium-79 Hydrolyzed • Babassuamidopropyl Betaine • Laureth-9 • Dimethicone Keratin • Behenamidopropyl Dimethyl- • Lauryl Betaine • Guar Hydroxypropyltrimo- • Quaternium-79 Hydrolyzed amine • Lauryl Glucoside nium Chloride Silk • Behenoyl PG-Trimonium • Lauryl Hydroxysultaine • Hydroxypropyl Guar • Sodium Lauroyl Glutamate Chloride • Lauryl Lactate Hydroxypropyltrimonium • Stearalkonium Chloride • Behenoyl PG-Trimonium Chlo- • Modified Oleamide DEA Chloride • Stearamidopropyl Dime- ride, Cetyl Alcohol • Oleamidopropyl Betaine • Isostearamidopropyl Eth- thylamine • Behentrimonium Chloride • Oleth-20, Ceteth-20 yldimonium Ethosulfate, • Sunflowerseedamidopro- • Capryl/Caprylic -

BETADET® BETADET® Anionic-Based Formulations
BETADET® S-20 BETADET® SHR VERY MILD SURFACTANT GREAT FORMULATION STABILITY ENHANCED FOAM VOLUME IMPROVED THICKENING EFFECT SULTAINES: MILD AMPHOTERIC SURFACTANTS FOR PERSONAL CARE KAO SURFACTANTS TECHNOLOGY AT YOUR SERVICE BETADET® S-20 & BETADET® SHR Foam, Viscosity & Mildness enhancers CHARACTERISTICS BETADET® S-20 and BETADET® SHR are recom- pH’s thus allowing their use in cosmetics formulations mended as secondary surfactants for very mild were alkali (i.e. depilatories) or acid media are neces- and high foaming products, achieving higher foam sary. Both products are also very stable to hard water, BETADET® S-20 and BETADET® volumes than alkyl betaines or alkyl amidopropyl improving the behavior of some sensitive anionic MAIN FEATURES betaines. These products are easier to thicken surfactants under extreme conditions. Additionally, SHR are very mild co-surfactants when combined with SLES, thus reducing the BETADET® S-20 shows a highlighted ability to reduce • Vegetable origin for personal care formulations. amount of electrolytes needed to obtain a desired the freezing point of final formulations making them • Preservative-free viscosity compared to other amphoteric surfactants. more stable at low temperatures, which helps to ob- Hydroxysultaines are highly effective amphoteric • Biodegradable Hydroxysultaines show excellent stability at extreme tain clear stable final products. Surfactants that improve the performance of final • Compatible with anionic, non-ionic and cationic cosmetic products. They are extremely mild cosur- surfactants -

Cocamidopropyl Betaine (CAPB)
Final Report to DWI September 2020 Personal Care Products and Domestic Cleaning Products – Toxicological Assessment of Prioritised List of Chemicals (Ref: DWI 70/2/331) FINAL REPORT Report to Defra/DWI IEH Consulting Ltd.|September 2020 Final Report to DWI September 2020 PCPs and DCPs – toxicological assessment of prioritised list of chemicals A Report to DWI by IEH Consulting Ltd. Disclaimer The views expressed in this report are those of the authors alone, representing their expert opinion based on the review of the available information and their understanding drawn from publications identified from targeted literature searches. Prepared by: Ruth Bevan, Sarah Bull Reviewed by: Camilla Alexander-White, Paul Rumsby Edited by: Paul Harrison September 2020 Final Report to DWI September 2020 Contents Contents Abbreviations ............................................................................................................................. 8 Executive Summary .................................................................................................................... 9 1.0 Introduction ....................................................................................................................... 11 1.1 Project Background ............................................................................................................ 11 1.2 Personal Care Products ...................................................................................................... 11 1.3 Domestic Cleaning Products ............................................................................................. -

Determination of SLES in Personal Care Products by Colloid Titration with Light Reflection Measurements
molecules Article Determination of SLES in Personal Care Products by Colloid Titration with Light Reflection Measurements Dorota Ziółkowska 1,* , Iryna Syrotynska 2 , Alexander Shyichuk 1 and Jan Lamkiewicz 1 1 Faculty of Chemical Technology and Engineering, UTP University of Science and Technology, Seminaryjna 3, 85-326 Bydgoszcz, Poland; [email protected] (A.S.); [email protected] (J.L.) 2 Biochemistry Department, Ivano-Frankivsk National Medical University, Halyts’ka 2, 76000 Ivano-Frankivs’k, Ukraine; [email protected] * Correspondence: [email protected] Abstract: The method of colloid titration with poly(diallyldimethylammonium) chloride has been improved to detect the endpoint with an off-vessel light reflectance sensor. The digital color sensor used measures light reflectance by means of light guides, with no immersion into the reaction solution. In such a method, the optical signal is free of disturbances caused by sticky flocs in the solution. The improved automatic titration set was applied for the determination of sodium laureth sulfate (SLES) in industrial batches and commercial personal care products. The sample color and opacity do not disturb the SLES quantification. When the SLES content lies in the range from 5% to 9%, the optimal sample weight is from 6 g to 3 g. Keywords: quantitation of surfactants; turbidity; polyDADMAC; PDADMAC; PDDA; cationic polymer Citation: Ziółkowska, D.; Syrotynska, I.; Shyichuk, A.; Lamkiewicz, J. Determination of SLES in Personal Care Products by Colloid 1. Introduction Titration with Light Reflection Sodium laureth sulfate (SLES) is a common primary surfactant in skin and hair Measurements. Molecules 2021, 26, care products [1–3]. Therefore, the determination of SLES concentration in personal care 2716. -

Product Catalog
Product Catalog Classic Surfactants Service and Quality Brought Together Classic Distributing has been selling surfactants for over 30 years! We have expanded our relationship with multiple toll manufacturers. This allows us to control pricing, quality, and consistency under our own brand. Now all of the high quality surfactants are made to our specifications to insure performance in the market place. We will continue to offer all of the services you have grown to expect from Classic Distributing including formulation knowledge and proprietary blend formulation. We also have capabilities with natural and non alcohol sulfate surfactant technologies that we have added in response to market demand. If you have special formulating requirements, just ask! Cosmetic Products AMIDES INCI DESCRIPTION Classic CE Cocamide DEA Classic KD Cocamide DEA Classic LSM Lauramide DEA (liquid) Classic CME Cocamide MEA (flake) Classic SD Soyamide DEA Classic B-MIPA Cocamide MIPA/Cocamidopropyl Betaine Classic DIPA Cocamide DIPA ALKYL ETHER SULFATES INCI DESCRIPTION Classic ES-2K Sodium Laureth Sulfate Classic ES-60 Sodium Laureth Sulfate Classic ES-70 Sodium Laureth Sulfate (2 mole) ALKYL SULFATES INCI DESCRIPTION Classic ALS Ammonium Lauryl Sulfate Classic TLS TEA-Lauryl Sulfate Classic SLS Sodium Lauryl Sulfate Classic SLN-95 Sodium Lauryl Sulfate Crystals AMINE OXIDES INCI DESCRIPTION Classic CAW Cocamidopropylamine Oxide Classic LO Lauramine Oxide AMPHOTERICS INCI DESCRIPTION Classic DCA Disodium Coco Amphodiacetate BETAINES INCI DESCRIPTION Classic CAS -

Coconut Oil Diethanolamine Condensate
COCONUT OIL DIETHANOLAMINE CONDENSATE 1. Exposure Data 1.1.2 Structural and molecular formulae and relative molecular mass 1.1 Chemical and physical data O CH2CH2OH Coconut oil diethanolamine condensate is CH3 (CH2)n CH2 C N a mixture of diethanolamides of the fatty acids that constitute coconut oil, which is composed CH2CH2OH of approximately 48.2% lauric acid (12:0), 18% myristic acid (14:0), 8.5% palmitic acid (16:0), 8% n = 5, 7, 9, 11, 13, 15 caprylic acid (8:0), 7% capric acid (10:0), 6% oleic acid (18:1, n-9), 2.3% stearic acid (18:0) and 2% C(7+n)H(15+2n)NO3 linoleic acid (18:2, n-6) (NTP, 2001). Relative molecular mass: range, 231–371 1.1.1 Nomenclature 1.1.3 Chemical and physical properties of the Chem. Abstr. Serv. Reg. No.: 68603-42-9 pure substance Deleted Chem. Abstr. Serv. Reg. Nos: 8036 Description: Clear, amber-coloured liquid 48-4; 8040-31-1; 8040-33-3; 12751-06-3; with a faint coconut odour (CTFA, 1986) 53028-62-9; 56448-72-7; 56832-66-7; 63091 Boiling-point: 169–275 °C 31-6; 66984-58-5; 67785-10-8; 67785-14-2; Melting-point: 23–35 °C (CTFA, 1986) 71343-51-6; 71343-71-0; 83652-14-6; 87714 Density: 0.99 g/cm3 at 20 °C (IUCLID, 18-9; 90651-47-1; 118104-13-5; 153189-69-6; 2000) 186615-78-1 (ChemID plus) Solubility: Miscible with water at 20 °C; Chem. Abstr. Name: Amides, coco, produces an alkali in aqueous solution N,N-bis(hydroxyethyl) (CTFA, 1986) Synonyms: N,N-Bis(hydroxyethyl)coco Octanol/water partition coefficient (P): amides; N,N-bis(hydroxyethyl)coco fatty log P, 3.52 (IUCLID, 2000) acid amides; cocamide DEA; -
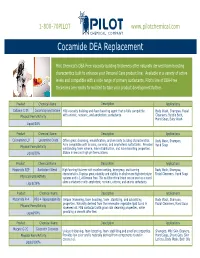
Cocamide DEA Replacement
1-800-70PILOT www.pilotchemical.com Cocamide DEA Replacement Pilot Chemical's DEA-Free viscosity building thickeners offer naturally derived foam boosting characteritics built to enhance your Personal Care product line. Available in a variety of active levels and compatible with a wide range of primary surfactants, Pilot's line of DEA-Free thickeners are readily formulated to take your product development further. Product Chemical Name Description Applications Caltaine C-35 Cocamidopropyl Betaine Mild viscosity building and foam boosting agent that is fully compatible Body Wash, Shampoo, Facial Physical Form/Activity with anionic, nonionic, and amphoteric surfactants. Cleansers, Bubble Bath, Hand Soap, Baby Wash Liquid/30% Product Chemical Name Description Applications Caloxamine LO Lauramine Oxide Offers great cleansing, emulsification, and viscosity building characteristics. Body Wash, Shampoo, Fully compatible with anionic, nonionic, and amphoteric surfactants. Provides Hand Soap Physical Form/Activity outstanding foam volume, foam stabiliaztion, and foam boosting properties. Liquid/30% Stable in low and high pH formulations. Product Chemical Name Description Applications Masamide REP Surfactant Blend High foaming thickener with excellent wetting, detergency, and foaming Body Wash, Shampoo, characteristics. Displays great solubility and stability in alkaline and high electrolyte Facial Cleansers, Hand Soap Physical Form/Activity systems and is 1,4-Dioxane Free. This multifunctional blend can be used as a stand Liquid/36% alone surfactant or with amphoteric, nonionic, cationic, and anionic surfactants. Product Chemical Name Description Applications Masamide R-4 PEG-4 Rapeseedamide Unique thickening, foam boosting, foam stabilizing, and solubilizing Body Wash, Shampoo, properties. Naturally derived from the renewable vegetable lipid found in Facial Cleansers, Hand Soap Physical Form/Activity rapeseed oil. -

Alternatives to Cocamide DEA California's Office of Environmental Health Hazard Assessment (OEHHA) Has Listed Cocamide DEA (CAS No
Alternatives to Cocamide DEA California's Office of Environmental Health Hazard Assessment (OEHHA) has listed Cocamide DEA (CAS No. 68603-42-9) on Proposition 65. For those looking to reformulate, Stepan is providing the following performance comparison and alternative surfactant recommendations to NINOL® 40-CO (1:1 Cocamide DEA) and NINOL® 11-CM (2:1 Cocamide DEA) for use in liquid dish detergents, all-purpose cleaners, degreasers, and vehicle care detergents. Stepan has a broad amphoteric product line and the technical expertise in hard surface care to assist our customers with product recommendation and reformulation. The recommended surfactants noted below take into consideration performance, handling, typical lead time, and estimated market price. If you require further assistance, please contact your local Stepan sales representative or Stepan U.S. Technical Service at [email protected] or (800) 745-7837. LIQUID DISH DETERGENTS (LDL) For an LDL economy formulation containing Linear Alkylbenzene Sulfonate (LAS) and Sodium Laureth Sulfate (SLES), the following surfactants showed equivalent or improved foam mileage and equivalent foam volume compared to NINOL® 40-CO on an equal actives basis. Recommendations Mixer Foam: LAS/SLES Economy AMMONYX® LMDO (Lauramidopropylamine Oxide) 160 AMPHOSOL® CG-50 (Cocamidopropyl Betaine) 150 AMMONYX® CDO SPECIAL (Cocamidopropylamine Oxide) 140 Shake Foam: LAS/SLES Economy 130 Soil D 500 120 Shell 400 110 Soil 300 100 90 200 Normalized Normalized Foam Mileage 80 100 Foam Volume (mL) 0 40-CO LMDO CG-50 CDO SPECIAL For an LDL premium formulation containing Sodium Lauryl Sulfate (SLS) and Sodium Laureth Sulfate (SLES), the following surfactants showed equivalent or improved foam mileage and equivalent foam volume compared to NINOL® 40-CO on an equal actives basis. -

Renewable Carbonindex
BIORENEWABLE CARBON INDEX Formulating green products is one of the current challenges facing developers of personal care and household cleaning REDUCED CARBON PRODUCTS products. SCT's Biorenewable Carbon Index can help make ingredient choices easier. CERTIFIED AND SUSTAINABLE Learn more at www.southern-chemical.com BIORENEWABLE CARBON INDEX Formulating green products is one of the current challenges facing developers of personal care and household cleaning products. SCT's Biorenewable Carbon Index can help make ingredient choices easier. % PALM SCT PRODUCT DESCRIPTION VEGETABLE % RCI FREE SCT-135 Sodium Lauryl Sulfate 65% 100% No SCT-1070 Sodium Laureth Sulfate 50% 76% No SCT-139 Potassium Alkanoate 79% 100% No SCT-140 Potassium Cocoate 80% 100% Yes SCT-722 Disodium Laureth Sulfosuccinate 36% 56% No SCT-725 Disodium Lauryl Sulfosuccinate 46% 76% No Sopal BT Bleach Thickener/Caustic Thickener Concentrate 80% 90% No Sopal JSB Mild Concentrate 24% 35% No Sopal Quat 1216 Sunflowerseedamidopropyl Dimethylamine Lactate 84% 89% Yes 95-100% Sopal Quat Rice Cocodimonium Hydroxypropyl Hydrolyzed Rice Protein 95% (Polymer) Yes Sopalex 222 Babassuamidopropyl betaine 52% 70% Yes Sopalex 340 Octyl Betaine 59% 67% No Sopalex 360-BET Cocamidopropyl Betaine 52% 70% Yes Sopalex 385 Coco-Betaine 69% 80% Yes Sopalex C37-HP Cocamidopropyl Betaine (stripped cut) 48% 66% No Sopalex OAB Caprylamidopropyl Betaine 44% 53% No Sopalteric CBS Cocamidopropyl Hydroxysultaine 57% 75% Yes Sopalteric CHS Cocamidopropyl Hydroxysultaine 55% 81% Yes Sopalteric CS Sodium