Myeloid Neoplasms Presentation Outline Overview of the Myeloid
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Updates in Mastocytosis
Updates in Mastocytosis Tryptase PD-L1 Tracy I. George, M.D. Professor of Pathology 1 Disclosure: Tracy George, M.D. Research Support / Grants None Stock/Equity (any amount) None Consulting Blueprint Medicines Novartis Employment ARUP Laboratories Speakers Bureau / Honoraria None Other None Outline • Classification • Advanced mastocytosis • A case report • Clinical trials • Other potential therapies Outline • Classification • Advanced mastocytosis • A case report • Clinical trials • Other potential therapies Mastocytosis symposium and consensus meeting on classification and diagnostic criteria for mastocytosis Boston, October 25-28, 2012 2008 WHO Classification Scheme for Myeloid Neoplasms Acute Myeloid Leukemia Chronic Myelomonocytic Leukemia Atypical Chronic Myeloid Leukemia Juvenile Myelomonocytic Leukemia Myelodysplastic Syndromes MDS/MPN, unclassifiable Chronic Myelogenous Leukemia MDS/MPN Polycythemia Vera Essential Thrombocythemia Primary Myelofibrosis Myeloproliferative Neoplasms Chronic Neutrophilic Leukemia Chronic Eosinophilic Leukemia, NOS Hypereosinophilic Syndrome Mast Cell Disease MPNs, unclassifiable Myeloid or lymphoid neoplasms Myeloid neoplasms associated with PDGFRA rearrangement associated with eosinophilia and Myeloid neoplasms associated with PDGFRB abnormalities of PDGFRA, rearrangement PDGFRB, or FGFR1 Myeloid neoplasms associated with FGFR1 rearrangement (EMS) 2017 WHO Classification Scheme for Myeloid Neoplasms Chronic Myelomonocytic Leukemia Acute Myeloid Leukemia Atypical Chronic Myeloid Leukemia Juvenile Myelomonocytic -

Mutation Analysis in Myeloproliferative Neoplasms AHS - M2101
Corporate Medical Policy Mutation Analysis in Myeloproliferative Neoplasms AHS - M2101 File Name: mutation_analysis_in_myeloproliferative_neoplasms Origination: 1/1/2019 Last CAP review: 8/2021 Next CAP review: 8/2022 Last Review: 8/2021 Description of Procedure or Service Myeloproliferative neoplasms (MPN) are a heterogeneous group of clonal disorders characterized by overproduction of one or more differentiated myeloid lineages (Grinfeld, Nangalia, & Green, 2017). These include polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (PMF). The majority of MPN result from somatic mutations in the 3 driver genes, JAK2, CALR, and MPL, which represent major diagnostic criteria in combination with hematologic and morphological abnormalities (Rumi & Cazzola, 2017). Related Policies: BCR-ABL 1 Testing for Chronic Myeloid Leukemia AHS-M2027 ***Note: This Medical Policy is complex and technical. For questions concerning the technical language and/or specific clinical indications for its use, please consult your physician. Policy BCBSNC will provide coverage for mutation analysis in myeloproliferative neoplasms when it is determined to be medically necessary because the medical criteria and guidelines shown below are met. Benefits Application This medical policy relates only to the services or supplies described herein. Please refer to the Member's Benefit Booklet for availability of benefits. Member's benefits may vary according to benefit design; therefore member benefit language should be reviewed before applying the terms of this medical policy. When Mutation Analysis in Myeloproliferative Neoplasms is covered 1. JAK2, CALR or MPL mutation testing is considered medically necessary for the diagnosis of patients presenting with clinical, laboratory, or pathological findings suggesting classic forms of myeloproliferative neoplasms (MPN), that is, polycythemia vera (PV), essential thrombocythemia (ET), or primary myelofibrosis (PMF) when ordered by a hematology and/or oncology specialist in the following situations: A. -

The Association of Bladder Myeloid Sarcoma and Unclassified
90 Case Report The association of bladder myeloid sarcoma and unclassified myelodysplastic/myeloproliferative disease Mesanede myeloid sarkom ve sınıflandırılamayan myeloproliferatif/myelodisplastik hastalık birlikteliği Mehmet Sönmez1, Ümit Çobanoğlu2, Sevdagül Mungan2, Bircan Sönmez3, Rasin Özyavuz4 1Department of Hematology, Karadeniz Technical University, School of Medicine, Trabzon, Turkey 2Department of Pathology, Karadeniz Technical University, School of Medicine, Trabzon, Turkey 3Department of Nuclear Medicine, Karadeniz Technical University, School of Medicine, Trabzon, Turkey 4Department of Urology, Karadeniz Technical University, School of Medicine, Trabzon, Turkey Abstract Myeloid sarcoma of the urinary bladder is a rare disorder. We report a 71-year-old man with hematuria who had a diffuse myeloid sarcoma of the bladder. He was also under follow-up for unclassified myeloproliferative/myelodysplastic disorder, diagnosed two months before. Abdominal ultrasonography and computed tomography findings were normal. Diagnostic cystoscopy revealed patchy areas of mucosal swelling with hyperemia. Histopathological examination of biopsies demon- strated a neoplasm composed of blasts showing myeloperoxidase positivity by immunohistochemistry. To our knowledge, the current case is the first case of myeloid sarcoma in the urinary bladder without evidence of a mass lesion, with a concurrent diagnosis of unclassifiable myelodysplastic/myeloproliferative disease. (Turk J Hematol 2009; 26: 90-2) Key words: Myeloid sarcoma, urinary bladder, unclassified myelodysplastic/myeloproliferative disease Received: April 3, 2008 Accepted: September 10, 2008 Özet Myeloid sarkom mesanede nadir görülen bir hastalıktır. Bu vaka takdiminde hematüri ile başvuran ve 2 ay önce sınıflandırılamayan myeloproliferatif/myelodisplastik hastalık tanısı almış 71 yaşında erkek hastada mesanede diffüz tutulum ile seyreden myeloid sarkom tanımlandı. Hastanın batın ultrasonografisi ve tomografisi normal olup, tanısal amaçlı sistosko- pide hiperemik ve ödemli bir mukoza izlendi. -

Acute Myeloid Leukemia Evolving from JAK 2-Positive Primary Myelofibrosis and Concomitant CD5-Negative Mantle Cell
Hindawi Publishing Corporation Case Reports in Hematology Volume 2012, Article ID 875039, 6 pages doi:10.1155/2012/875039 Case Report Acute Myeloid Leukemia Evolving from JAK 2-Positive Primary Myelofibrosis and Concomitant CD5-Negative Mantle Cell Lymphoma: A Case Report and Review of the Literature Diana O. Treaba,1 Salwa Khedr,1 Shamlal Mangray,1 Cynthia Jackson,1 Jorge J. Castillo,2 and Eric S. Winer2 1 Department of Pathology and Laboratory Medicine, Rhode Island Hospital, The Warren Alpert Medical School, Brown University, Providence, RI 02903, USA 2 Division of Hematology/Oncology, The Miriam Hospital, The Warren Alpert Medical School, Brown University, Providence, RI 02904, USA Correspondence should be addressed to Diana O. Treaba, [email protected] Received 2 April 2012; Accepted 21 June 2012 Academic Editors: E. Arellano-Rodrigo, G. Damaj, and M. Gentile Copyright © 2012 Diana O. Treaba et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Primary myelofibrosis (formerly known as chronic idiopathic myelofibrosis), has the lowest incidence amongst the chronic myeloproliferative neoplasms and is characterized by a rather short median survival and a risk of progression to acute myeloid leukemia (AML) noted in a small subset of the cases, usually as a terminal event. As observed with other chronic myeloproliferative neoplasms, the bone marrow biopsy may harbor small lymphoid aggregates, often assumed reactive in nature. In our paper, we present a 70-year-old Caucasian male who was diagnosed with primary myelofibrosis, and after 8 years of followup and therapy developed an AML. -

Mutations and Prognosis in Primary Myelofibrosis
Leukemia (2013) 27, 1861–1869 & 2013 Macmillan Publishers Limited All rights reserved 0887-6924/13 www.nature.com/leu ORIGINAL ARTICLE Mutations and prognosis in primary myelofibrosis AM Vannucchi1, TL Lasho2, P Guglielmelli1, F Biamonte1, A Pardanani2, A Pereira3, C Finke2, J Score4, N Gangat2, C Mannarelli1, RP Ketterling5, G Rotunno1, RA Knudson5, MC Susini1, RR Laborde5, A Spolverini1, A Pancrazzi1, L Pieri1, R Manfredini6, E Tagliafico7, R Zini6, A Jones4, K Zoi8, A Reiter9, A Duncombe10, D Pietra11, E Rumi11, F Cervantes12, G Barosi13, M Cazzola11, NCP Cross4 and A Tefferi2 Patient outcome in primary myelofibrosis (PMF) is significantly influenced by karyotype. We studied 879 PMF patients to determine the individual and combinatorial prognostic relevance of somatic mutations. Analysis was performed in 483 European patients and the seminal observations were validated in 396 Mayo Clinic patients. Samples from the European cohort, collected at time of diagnosis, were analyzed for mutations in ASXL1, SRSF2, EZH2, TET2, DNMT3A, CBL, IDH1, IDH2, MPL and JAK2. Of these, ASXL1, SRSF2 and EZH2 mutations inter-independently predicted shortened survival. However, only ASXL1 mutations (HR: 2.02; Po0.001) remained significant in the context of the International Prognostic Scoring System (IPSS). These observations were validated in the Mayo Clinic cohort where mutation and survival analyses were performed from time of referral. ASXL1, SRSF2 and EZH2 mutations were independently associated with poor survival, but only ASXL1 mutations held their prognostic relevance (HR: 1.4; P ¼ 0.04) independent of the Dynamic IPSS (DIPSS)-plus model, which incorporates cytogenetic risk. In the European cohort, leukemia-free survival was negatively affected by IDH1/2, SRSF2 and ASXL1 mutations and in the Mayo cohort by IDH1 and SRSF2 mutations. -

Myelofibrosis (MF)
Myelofibrosis (MF) A Guide for Patients Introduction Being diagnosed with myelofibrosis (MF) can be a shock, particularly when you may have never heard of it. If you have questions about MF – what causes it, who it affects, how it affects your body, what symptoms to expect and likely treatments – this booklet covers the basics for you. You will also find useful advice Haematologist at University about how to get the best from Hospital of Wales, Cardiff. We your haematologist, plus practical are also grateful to Chris Rogers, advice on how to help important patient reviewer, for his valuable people in your life understand contribution. The rewrite was put such a rare condition. For together by Lisa Lovelidge and more information talk to your peer reviewed by Professor Claire haematologist or clinical nurse Harrison. This booklet has since specialist. been updated by our Patient Information Writer Isabelle Leach This booklet originally written and peer reviewed by Dr Sebastian by Professor Claire Harrison, Francis. We also appreciate Consultant Haematologist Norman Childs and Amy Cross for at Guy’s and St Thomas’ their input as patient reviewers NHS Foundation Trust, and as well as Samantha Robertson subsequently revised by Dr whose husband had MF. Steve Knapper, Consultant If you would like any information on the sources used for this booklet, please email [email protected] for a list of references. Version 4 Printed: 10/2020 2 www.leukaemiacare.org.uk Review date: 10/2022 In this booklet Introduction 2 In this booklet 3 About Leukaemia Care 4 What is myelofibrosis? 6 What are the signs and symptoms of MF? 9 How is MF diagnosed? 10 What is the treatment for MF? 12 Living with MF 26 Talking about MF 28 Glossary 31 Useful contacts and further support 39 Helpline freephone 08088 010 444 3 About Leukaemia Care Leukaemia Care is a national charity dedicated to ensuring that people affected by blood cancer have access to the right information, advice and support. -

Acute Massive Myelofibrosis with Acute Lymphoblastic Leukemia Akut Masif Myelofibrozis Ve Akut Lenfoblastik Lösemi Birlikteliği
204 Case Report Acute massive myelofibrosis with acute lymphoblastic leukemia Akut masif myelofibrozis ve akut lenfoblastik lösemi birlikteliği Zekai Avcı1, Barış Malbora1, Meltem Gülşan1, Feride Iffet Şahin2, Bülent Celasun3, Namık Özbek1 1Department of Pediatrics, Başkent University Faculty of Medicine, Ankara, Turkey 2Department of Medical Genetics, Başkent University Faculty of Medicine, Ankara, Turkey 3Department of Pathology, Başkent University Faculty of Medicine, Ankara, Turkey Abstract Acute myelofibrosis is characterized by pancytopenia of sudden onset, megakaryocytic hyperplasia, extensive bone mar- row fibrosis, and the absence of organomegaly. Acute myelofibrosis in patients with acute lymphoblastic leukemia is extremely rare. We report a 4-year-old boy who was diagnosed as having acute massive myelofibrosis and acute lym- phoblastic leukemia. Performing bone marrow aspiration in this patient was difficult (a “dry tap”), and the diagnosis was established by means of a bone marrow biopsy and immunohistopathologic analysis. The prognostic significance of acute myelofibrosis in patients with acute lymphoblastic leukemia is not clear. (Turk J Hematol 2009; 26: 204-6) Key words: Acute myelofibrosis, acute lymphoblastic leukemia, dry tap Received: April 9, 2008 Accepted: December 24, 2008 Özet Akut myelofibrozis ani gelişen pansitopeni, kemik iliğinde megakaryositik hiperplazi, belirgin fibrozis ve organomegali olmaması ile karakterize bir hastalıktır. Akut myelofibrozis ile akut lenfoblastik lösemi birlikteliği çok nadir görülmektedir. -

Treatment Outcomes of Pediatric Acute Myeloid Leukemia In
children Article Treatment Outcomes of Pediatric Acute Myeloid Leukemia in the Yeungnam Region: A Multicenter Retrospective Study of the Study Alliance of Yeungnam Pediatric Hematology–Oncology (SAYPH) Jae Min Lee 1 , Eu Jeen Yang 2 , Kyung Mi Park 2 , Young-Ho Lee 3, Heewon Chueh 4, Jeong Ok Hah 5, Ji Kyoung Park 6, Jae Young Lim 7, Eun Sil Park 7, Sang Kyu Park 8, Heung Sik Kim 9, Ye Jee Shim 10 , Jeong A. Park 11,12, Eun Jin Choi 13, Kun Soo Lee 14, Ji Yoon Kim 14 and Young Tak Lim 2,* 1 Department of Pediatrics, College of Medicine, Yeungnam University, Daegu 42415, Korea; [email protected] 2 Department of Pediatrics, Pusan National University Children’s Hospital, Pusan National University, School of Medicine, Yangsan 50612, Korea; [email protected] (E.J.Y.); [email protected] (K.M.P.) 3 Department of Pediatrics, Hanyang University College of Medicine, Hanyang University Medical Center, Seoul 04763, Korea; [email protected] 4 Department of Pediatrics, Dong-A University College of Medicine, Busan 49201, Korea; [email protected] 5 Department of Pediatrics, Daegu Fatima Hospital, Daegu 41199, Korea; [email protected] 6 Department of Pediatrics, Inje University College of Medicine, Busan Paik Hospital, Busan 47392, Korea; [email protected] 7 Department of Pediatrics, Gyeongsang National University College of Medicine, Jinju 52727, Korea; [email protected] (J.Y.L.); [email protected] (E.S.P.) Citation: Lee, J.M.; Yang, E.J.; Park, 8 Department of Pediatrics, Ulsan University Hospital, Ulsan 44033, Korea; [email protected] K.M.; Lee, Y.-H.; Chueh, H.; Hah, J.O.; 9 Department of Pediatrics, Keimyung University School of Medicine, Keimyung University Daegu Dongsan Park, J.K.; Lim, J.Y.; Park, E.S.; Park, Hospital, Daegu 41931, Korea; [email protected] S.K.; et al. -
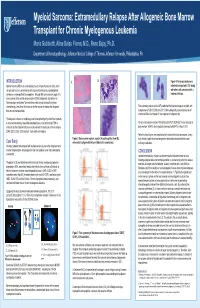
Myeloid Sarcoma
Myeloid Sarcoma: Extramedullary Relapse After Allogeneic Bone Marrow Transplant for Chronic Myelogenous Leukemia Maria Gubbiotti, Alina Dulau Florea, M.D., Renu Bajaj, Ph.D. Department of Hematopathology, Jefferson Medical College of Thomas Jefferson University, Philadelphia, PA INTRODUCTION A. B. Figure 4: Numerous blasts were Myeloid sarcoma (MS) is an extramedullary tumor of myeloid precursor cells, which detected in the patient’s CSF along can precede or occur concomitantly with acute myeloid leukemia, myelodysplastic with other cells consistent with a syndrome, or myeloproliferative neoplasms. Although MS can involve any organ, it is leukemic infiltrate. more common in the central nervous system (CNS) and gonads, sites known as “pharmacologic sanctuaries” where leukemic cells can survive despite systemic chemotherapy. Less often, this tumor can be the manner of relapse after allogeneic Flow cytometry analysis of the CSF established the blast phenotype as myeloid, with bone marrow transplantation. coexpression of CD13, CD33 and CD117. She subsequently received two cycles of intrathecal ARA-C and repeat LP was negative for malignant cells. The diagnosis is based on morphology and immunophenotype by either flow cytometry or immunohistochemistry of paraffin-embedded tissue, and confirmed by FISH or Reverse transcription real-time PCR detected the P210 BCR-ABL1 fusion transcript in molecular studies. Myeloid sarcomas usually express the leukocyte common antigens bone marrow : 0.064%, which gradually increased to 98.947% in March 2013. CD45, CD13, CD33, CD43 and lack T-cell and B-cell antigens. Patient’s clinical course was complicated with multiple infections (pneumonia, urinary Figure 2: Bone marrow aspirate, regular (A) and magnified view (B), tract infection) septic shock and progressive deterioration despite antibiotics and Case Study demonstrating hypercellularity and blast crisis respectively. -

The Evolving Understanding of Prognosis in Post–Essential Thrombocythemia Myelofibrosis and Post–Polycythemia Vera Myelofibrosis Vs Primary Myelofibrosis
The Evolving Understanding of Prognosis in Post–Essential Thrombocythemia Myelofibrosis and Post–Polycythemia Vera Myelofibrosis vs Primary Myelofibrosis Lucia Masarova, MD, and Srdan Verstovsek, MD Dr Masarova is an assistant professor Abstract: Myelofibrosis (MF) is the most aggressive of the classic and Dr Verstovsek is a professor in Philadelphia chromosome–negative myeloproliferative neoplasms the Department of Leukemia at The (MPNs). In some patients with essential thrombocytopenia or poly- University of Texas MD Anderson cythemia vera, which are relatively benign MPNs, MF develops Cancer Center in Houston, Texas. as a natural evolution of their disease, resulting in post–essential thrombocythemia myelofibrosis (PET-MF) or post–polycythemia Corresponding author: vera myelofibrosis (PPV-MF). Presenting with the same clini- Srdan Verstovsek, MD, PhD cal features, including debilitating symptoms and signs of bone MD Anderson Cancer Center marrow failure, PET/PPV-MF has traditionally been considered Department of Leukemia akin to primary myelofibrosis (PMF). However, recent observa- 1515 Holcombe Blvd, Unit 428 Houston, TX 77030 tions that PET/PPV-MF may be a distinct clinical entity from PMF Tel: (713) 745-3429 have triggered efforts to improve prognostication in these diseases. E-mail: [email protected] Novel predictive models that incorporate rapidly emerging clini- cal and molecular data are being developed to improve outcomes in patients with PMF or PET/PPV-MF. This review focuses on the major clinical features and prognostic classification systems used in PMF and PET/PPV-MF. Introduction Myelofibrosis (MF) is one of the chronic Philadelphia chromosome– negative myeloproliferative neoplasms (MPNs). It is characterized by the clonal proliferation of myeloid cells, leading to extramedullary hematopoiesis, hepatosplenomegaly, constitutional symptoms (ie, fatigue, night sweats, weight loss, and fever), and cytopenia, along with bone marrow fibrosis and an increased risk for evolution into acute myeloid leukemia (AML). -

Compressive Myelopathy Caused by Isolated Epidural Myeloid Sarcoma with Systemic Mastocytosis
Clinical Notes Compressive myelopathy caused by isolated epidural myeloid sarcoma with systemic mastocytosis. Rare presentation of a hematological malignancy Cyril J. Kurian, MBBS, Indira Madhavan, MBBS, MD, Prabhalakshmi K. Krishnankutty, MBBS, MD, Mekkattukunnel A. Andrews, MD, DM. eurological manifestations of acute leukemia are Ndue to direct involvement by meningeal infiltration and myeloid sarcoma; and indirect involvement by immunosuppression and treatment related side effects. It is rare for myeloid sarcoma to present without bone marrow involvement (isolated myeloid sarcoma or primary granulocytic sarcoma).1 It is even rarer for an isolated myeloid sarcoma to present in the epidural space. We evaluated a case of paraplegia admitted to our department. He had several atypical features that we would like to present in this report. A 39-year-old gentleman with a body weight of Figure 1 - Magnetic resonance imaging of thoracic spine T1W sagittal 58 kg presented with paresthesia and heaviness of view, arrow showing extradural mass at T6 level. both lower limbs of 4 days duration. He was found to have spastic paraplegia with bladder involvement and sensory level at T6. The clinical diagnosis of acute peripheral blood picture showed dimorphic anemia, transverse myelitis was made. Table 1 summarizes the occasional large cells with granular cytoplasm and nucleus with condensed chromatin, and no blast cells. laboratory investigations. The MRI study of the dorsal Ultrasound of the abdomen showed mild splenomegaly. spine (Figure 1) shows that a moderate sized enhancing Urine Bence Jones protein was absent. No M band was posterior epidural component was compressing the seen on serum protein electrophoresis. Bone marrow thecal sac and spinal cord. -

Chronic Myeloid Leukemia Mimicking Primary Myelofibrosis: a Case Report
ISSN: 2640-7914 DOI: https://dx.doi.org/10.17352/ahcrr CLINICAL GROUP Received: 02 November, 2020 Case Report Accepted: 01 February, 2021 Published: 02 February, 2021 *Corresponding author: Anju S, Junior Resident, Gov- Chronic myeloid leukemia ernment Medical College, Kottayam, Kerala, India, Tel: 91 9496057350; E-mail: mimicking primary Keywords: Chronic myeloid leukemia; Primary myelo- fi brosis; Blast crisis; Myeloproliferative neoplasms myelofi brosis: A case report https://www.peertechz.com Anju S*, Jayalakshmy PL and Sankar Sundaram Junior Resident, Government Medical College, Kottayam, Kerala, India Abstract Bone marrow fi brosis leading to dry tap aspiration and often associated with blast crisis has previously been reported in both Chronic myeloid leukemia and Primary myelofi brosis. The similarities between these two conditions in terms of clinical presentation and morphology can really create a diagnostic dilemma. Here we present a case of Chronic myeloid leukemia in fi brosis and blast crisis in a 32 year old lady which closely resembled Primary myelofi brosis in transformation. All myeloproliferative neoplasms can undergo blast transformation. In this case, the detection of Philadelphia chromosome helped to distinguish Chronic myeloid leukemia from other myeloproliferative neoplasms. Introduction diffi cult to distinguish the different MPNs especially those in blast crisis and fi brosis. The treatment of CML involves targeted Myeloproliferative Neoplasm (MPN) results from therapy namely, Imatinib mesylate, which is a tyrosine kinase unchecked proliferation of the cellular elements in the bone inhibitor. Also, JAK1/2 inhibitors administered in PMF patients marrow characterized by panmyelosis and accompanied by show clinical improvement [2]. So, it is Important to distinguish erythrocytosis, granulocytosis, and/or thrombocytosis in every MPNs even in their blastic phase as they have different the peripheral blood.