Chapter 1: Introduction
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

APPLE (Fruit Varieties)
E TG/14/9 ORIGINAL: English DATE: 2005-04-06 INTERNATIONAL UNION FOR THE PROTECTION OF NEW VARIETIES OF PLANTS GENEVA * APPLE (Fruit Varieties) UPOV Code: MALUS_DOM (Malus domestica Borkh.) GUIDELINES FOR THE CONDUCT OF TESTS FOR DISTINCTNESS, UNIFORMITY AND STABILITY Alternative Names:* Botanical name English French German Spanish Malus domestica Apple Pommier Apfel Manzano Borkh. The purpose of these guidelines (“Test Guidelines”) is to elaborate the principles contained in the General Introduction (document TG/1/3), and its associated TGP documents, into detailed practical guidance for the harmonized examination of distinctness, uniformity and stability (DUS) and, in particular, to identify appropriate characteristics for the examination of DUS and production of harmonized variety descriptions. ASSOCIATED DOCUMENTS These Test Guidelines should be read in conjunction with the General Introduction and its associated TGP documents. Other associated UPOV documents: TG/163/3 Apple Rootstocks TG/192/1 Ornamental Apple * These names were correct at the time of the introduction of these Test Guidelines but may be revised or updated. [Readers are advised to consult the UPOV Code, which can be found on the UPOV Website (www.upov.int), for the latest information.] i:\orgupov\shared\tg\applefru\tg 14 9 e.doc TG/14/9 Apple, 2005-04-06 - 2 - TABLE OF CONTENTS PAGE 1. SUBJECT OF THESE TEST GUIDELINES..................................................................................................3 2. MATERIAL REQUIRED ...............................................................................................................................3 -

CMI Orchards Expansion a Boon for Retailers
- Advertisement - CMI Orchards expansion a boon for retailers August 12, 2020 Furthering its commitment to being a one-stop-shop for retailers looking for premium apples, pears and cherries grown in Washington state, CMI Orchards LLC reports continued expansion for the company. Strategic partnerships with Yakima Fruit, as well as with Pine Canyon Growers, in addition to significant internal growth, has added over 4 million boxes to CMI’s manifest. “This is all a part of a long-range plan for strategic growth to better serve our customers,” said CMI President Bob Mast. “The design began to unfold back in 2018 when CMI Orchards added Pine Canyon Growers as a grower, packer and shipper. This addition greatly strengthened our manifest and our progress has enabled us to team up with another great partner in Yakima Fruit.” “Yakima Fruit, formerly connected to Yakima Fresh, has a highly desirable manifest that greatly bolsters our offerings, including exceptional early Honeycrisp and high-colored genetic strains of Honeycrisp, Gala and Fuji,” said Mast. “The partnership also adds considerable Cosmic Crisp volume.” The partnership was executed on March 20. As a result of recent growth, CMI reports the following increases in its core, organic and branded offerings: Granny Smith (89 percent), Gala (44 percent), Pink Lady (92 percent), Fuji (62 percent), Honeycrisp (62 percent), and organic Honeycrisp (47 percent). According to CMI, its total organic crop has surpassed 3.5 million boxes, which is made up of increases in core organic varieties such as Gala, Fuji and Honeycrisp, in addition to branded organics, which create opportunities for retail pricing tiers that help boost sales. -
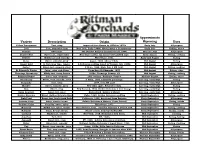
Variety Description Origin Approximate Ripening Uses
Approximate Variety Description Origin Ripening Uses Yellow Transparent Tart, crisp Imported from Russia by USDA in 1870s Early July All-purpose Lodi Tart, somewhat firm New York, Early 1900s. Montgomery x Transparent. Early July Baking, sauce Pristine Sweet-tart PRI (Purdue Rutgers Illinois) release, 1994. Mid-late July All-purpose Dandee Red Sweet-tart, semi-tender New Ohio variety. An improved PaulaRed type. Early August Eating, cooking Redfree Mildly tart and crunchy PRI release, 1981. Early-mid August Eating Sansa Sweet, crunchy, juicy Japan, 1988. Akane x Gala. Mid August Eating Ginger Gold G. Delicious type, tangier G Delicious seedling found in Virginia, late 1960s. Mid August All-purpose Zestar! Sweet-tart, crunchy, juicy U Minn, 1999. State Fair x MN 1691. Mid August Eating, cooking St Edmund's Pippin Juicy, crisp, rich flavor From Bury St Edmunds, 1870. Mid August Eating, cider Chenango Strawberry Mildly tart, berry flavors 1850s, Chenango County, NY Mid August Eating, cooking Summer Rambo Juicy, tart, aromatic 16th century, Rambure, France. Mid-late August Eating, sauce Honeycrisp Sweet, very crunchy, juicy U Minn, 1991. Unknown parentage. Late Aug.-early Sept. Eating Burgundy Tart, crisp 1974, from NY state Late Aug.-early Sept. All-purpose Blondee Sweet, crunchy, juicy New Ohio apple. Related to Gala. Late Aug.-early Sept. Eating Gala Sweet, crisp New Zealand, 1934. Golden Delicious x Cox Orange. Late Aug.-early Sept. Eating Swiss Gourmet Sweet-tart, juicy Switzerland. Golden x Idared. Late Aug.-early Sept. All-purpose Golden Supreme Sweet, Golden Delcious type Idaho, 1960. Golden Delicious seedling Early September Eating, cooking Pink Pearl Sweet-tart, bright pink flesh California, 1944, developed from Surprise Early September All-purpose Autumn Crisp Juicy, slow to brown Golden Delicious x Monroe. -

Apples: Organic Production Guide
A project of the National Center for Appropriate Technology 1-800-346-9140 • www.attra.ncat.org Apples: Organic Production Guide By Tammy Hinman This publication provides information on organic apple production from recent research and producer and Guy Ames, NCAT experience. Many aspects of apple production are the same whether the grower uses low-spray, organic, Agriculture Specialists or conventional management. Accordingly, this publication focuses on the aspects that differ from Published nonorganic practices—primarily pest and disease control, marketing, and economics. (Information on March 2011 organic weed control and fertility management in orchards is presented in a separate ATTRA publica- © NCAT tion, Tree Fruits: Organic Production Overview.) This publication introduces the major apple insect pests IP020 and diseases and the most effective organic management methods. It also includes farmer profiles of working orchards and a section dealing with economic and marketing considerations. There is an exten- sive list of resources for information and supplies and an appendix on disease-resistant apple varieties. Contents Introduction ......................1 Geographical Factors Affecting Disease and Pest Management ...........3 Insect and Mite Pests .....3 Insect IPM in Apples - Kaolin Clay ........6 Diseases ........................... 14 Mammal and Bird Pests .........................20 Thinning ..........................20 Weed and Orchard Floor Management ......20 Economics and Marketing ........................22 Conclusion -

Fruit, Nut & Grape Varieties for the Contra Costa Home Orchard
ccmg.ucanr.edu February 2020 Fruit, Nut & Grape Varieties for the Contra Costa Home Orchard by Janet Caprile, Contra Costa County Farm Advisor Emeritus NOTES: The County has been divided into 4 climate zones based on those outlined in the Sunset Western Garden Book. The zones include: Zone 17: Coastal strips Kensington San Pablo Rodeo (bayside) El Cerrito Pinole (bayside) Crockett Richmond Hercules (bayside) Zone 16: Northern California coast thermal belts Orinda (far west) Zone 15: Chilly winters areas along the Coast Range Orinda (central) Martinez (central & west) Walnut Creek (most) El Sobrante Pacheco Alamo (east of Hwy 680) Pinole (inland) Pleasant Hill Danville ( most) Hercules (inland) Concord (most) Rodeo (inland) Clayton Zone 14: Northern California’s inland area with some ocean influence Pittsburg Orinda (east) Alamo (west of Hwy 680) Antioch Moraga Danville (part) Oakley Lafayette Blackhawk Brentwood Walnut Creek (west of Hwy 680) San Ramon Discovery Bay Concord (part) Byron Martinez ( east) Refer to this Sunset website to find your “zone”: https://www.sunset.com/garden/climate-zones/sunset-climate-zone- bay-area LEGEND: COMMONLY GROWN AND COMMONLY AVAILABLE VARIETIES SHOWN IN BOLDFACE TYPE. Parentheses indicate zones that may support the listed fruit variety but are not ideal. v-2020-02-27 1 of 18 The University of California prohibits discrimination or harassment of any person in any of its programs or activities. See the complete Nondiscrimination Statement at ucanr.edu. ccmg.ucanr.edu Fruit, Nut & Grape Varieties for the Contra Costa Home Orchard February 2020 ALMOND Almonds have a low chill requirement (200-300 hours) but need summer heat to mature a crop. -

Handling of Apple Transport Techniques and Efficiency Vibration, Damage and Bruising Texture, Firmness and Quality
Centre of Excellence AGROPHYSICS for Applied Physics in Sustainable Agriculture Handling of Apple transport techniques and efficiency vibration, damage and bruising texture, firmness and quality Bohdan Dobrzañski, jr. Jacek Rabcewicz Rafa³ Rybczyñski B. Dobrzañski Institute of Agrophysics Polish Academy of Sciences Centre of Excellence AGROPHYSICS for Applied Physics in Sustainable Agriculture Handling of Apple transport techniques and efficiency vibration, damage and bruising texture, firmness and quality Bohdan Dobrzañski, jr. Jacek Rabcewicz Rafa³ Rybczyñski B. Dobrzañski Institute of Agrophysics Polish Academy of Sciences PUBLISHED BY: B. DOBRZAŃSKI INSTITUTE OF AGROPHYSICS OF POLISH ACADEMY OF SCIENCES ACTIVITIES OF WP9 IN THE CENTRE OF EXCELLENCE AGROPHYSICS CONTRACT NO: QLAM-2001-00428 CENTRE OF EXCELLENCE FOR APPLIED PHYSICS IN SUSTAINABLE AGRICULTURE WITH THE th ACRONYM AGROPHYSICS IS FOUNDED UNDER 5 EU FRAMEWORK FOR RESEARCH, TECHNOLOGICAL DEVELOPMENT AND DEMONSTRATION ACTIVITIES GENERAL SUPERVISOR OF THE CENTRE: PROF. DR. RYSZARD T. WALCZAK, MEMBER OF POLISH ACADEMY OF SCIENCES PROJECT COORDINATOR: DR. ENG. ANDRZEJ STĘPNIEWSKI WP9: PHYSICAL METHODS OF EVALUATION OF FRUIT AND VEGETABLE QUALITY LEADER OF WP9: PROF. DR. ENG. BOHDAN DOBRZAŃSKI, JR. REVIEWED BY PROF. DR. ENG. JÓZEF KOWALCZUK TRANSLATED (EXCEPT CHAPTERS: 1, 2, 6-9) BY M.SC. TOMASZ BYLICA THE RESULTS OF STUDY PRESENTED IN THE MONOGRAPH ARE SUPPORTED BY: THE STATE COMMITTEE FOR SCIENTIFIC RESEARCH UNDER GRANT NO. 5 P06F 012 19 AND ORDERED PROJECT NO. PBZ-51-02 RESEARCH INSTITUTE OF POMOLOGY AND FLORICULTURE B. DOBRZAŃSKI INSTITUTE OF AGROPHYSICS OF POLISH ACADEMY OF SCIENCES ©Copyright by BOHDAN DOBRZAŃSKI INSTITUTE OF AGROPHYSICS OF POLISH ACADEMY OF SCIENCES LUBLIN 2006 ISBN 83-89969-55-6 ST 1 EDITION - ISBN 83-89969-55-6 (IN ENGLISH) 180 COPIES, PRINTED SHEETS (16.8) PRINTED ON ACID-FREE PAPER IN POLAND BY: ALF-GRAF, UL. -

Effect of Cultivar, Position of Fruits in Tree-Crown and of Summer Pruning on Surface Temperature of Apples and Pears, Ejpau, 15(2), #03
Electronic Journal of Polish Agricultural Universities (EJPAU) founded by all Polish Agriculture Universities presents original papers and review articles relevant to all aspects of agricultural sciences. It is target for persons working both in science and industry, regulatory agencies or teaching in agricultural sector. Covered by IFIS Publishing (Food Science and Technology Abstracts), ELSEVIER Science - Food Science and Technology Program, CAS USA (Chemical Abstracts), CABI Publishing UK and ALPSP (Association of Learned and Professional Society Publisher - full membership). Presented in the Master List of Thomson ISI. ELECTRONIC 2012 JOURNAL Volume 15 OF POLISH Issue 2 AGRICULTURAL Topic HORTICULTURE UNIVERSITIES Copyright © Wydawnictwo Uniwersytetu Przyrodniczego we Wrocławiu, ISSN 1505-0297 LIPA T., LIPECKI J., JANISZ A., 2012. EFFECT OF CULTIVAR, POSITION OF FRUITS IN TREE-CROWN AND OF SUMMER PRUNING ON SURFACE TEMPERATURE OF APPLES AND PEARS, EJPAU, 15(2), #03. Available Online http://www.ejpau.media.pl EFFECT OF CULTIVAR, POSITION OF FRUITS IN TREE-CROWN AND OF SUMMER PRUNING ON SURFACE TEMPERATURE OF APPLES AND PEARS Tomasz Lipa, Janusz Lipecki, Anna Janisz Departament of Pomology, University of Life Sciences in Lublin, Poland ABSTRACT Extensive studies (2006–2009) on the influence of apple and pear fruit surface temperature, in dependence on the fruit position in tree canopy and summer pruning, were conducted in a commercial orchard in Lublin district, Poland. There was a significant effect of fruit position in the canopy on heat accumulation in fruit surface. Fruits born in southern part of the canopy were more heated than those from other tree parts. It was found that a blush contributed to an increase of fruit temperature, especially those from southern parts of the canopy. -

SPECIAL ORDER FRUIT TREE LIST 2018 Orders from This List Must Be Pre-Paid and Placed No Later Than November 5Th, 2017
SPECIAL ORDER FRUIT TREE LIST 2018 Orders from this list must be pre-paid and placed no later than November 5th, 2017. Pre-season discounts do not apply, and all sales are final. In addition to our regular fruit tree list, our supplier, Dave Wilson Nursery has opened their entire inventory for you to select from. For descriptions of specific varieties you may visit their web site, davewilson.com. Each variety is listed with all available rootstocks. If you have a preference, please specify it when ordering, otherwise we’ll choose a rootstock for you. Rootstock information can be found on the last page of this list. Please note that all deciduous fruit trees may be kept to any chosen height by proper winter and summer pruning. You may refer to our Gro-Sheet, Growing a Successful Backyard Orchard for more information. Almonds, Semi-Dwarf $49.99 Jonathan – M111 Chinese – Citation, St. Julian All-in One – Marianna, Nemaguard King (Tompkins King) – M111 Earli-Autumn - Citation Garden Prince – Nemaguard Liberty – Geneva, M7 Flavor Giant - Citation McIntosh – M7, M111 Flora Gold – Citation Almonds, Standard $49.99 Melrose – M7 Goldcot – St. Julian Butte – Lovell Mollie’s Delicious – M111 Gold Kist - Citation Carmel – Nemaguard Mutsu (Crispin) – M111 Harcot – Citation, St. Julian Neplus – Lovell, Nemaguard Northern Spy - M7 Harglow – Citation, St. Julian Nonpareil – Lovell, Nemaguard, Pettingill – M111 Hunza - Citation Peerless – Lovell Pink Lady – M27, Geneva, M7, M111 Katy – Citation Price – Nemaguard Pink Pearl – M111 Mokel – Marianna Red -

Apple Anna, 200 Chill Hours Temperate Fruit Dorsett Golden
Temperate Fruit Apple Anna, 200 chill hours Anna apple is a dual purpose apple that is very early ripening and does well in warm climates. Anna was bred by Abba Stein at the Ein Shemer kibbutz in Israel, in order to achieve a Golden Delicious-like apple, that can be cultivated in nearly tropical areas. Sweet, crisp, ripens in late June. Excellent for eating or cooking Dorsett Golden, 100 chill hours Golden Dorsett produces a medium sized, firm, and sweet apple perfect for eating fresh off the tree. The apples, a soft yellow with a pink blush, ripen in late June or July, and after picked, they can be kept for two weeks if refrigerated. The Golden Dorsett is perfect for Gulf Coast planting. Ein Shemer, 250 chill hours The Ein Shemer Apple produces a pale yellow, medium-sized apple. The apple's sweet, semi-acidic taste is perfect for eating right off the tree or for making into applesauce or pie. At maturity, the Ein Shemer apple can reach a height and width of 12-15 feet. Ripe in July. Fuji, 250-350 chill hours Crisp and sweet, ripens in June, the Fuji apple is a small to medium size fruit with a reddish pink over yellow appearance. Apple trees require well drained soil but will grow in clay or sandy soil. Multi-graft Apple 7 gallon (FBMG does not know yet if we will receive these. Will update soon.) Two or more varieties grafted onto one rootstock. These specimens are perfect for smaller gardens where a variety of flavors and an extended harvest season is desired. -

Using Whole-Genome SNP Data to Reconstruct a Large Multi
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Archivio istituzionale della ricerca - Alma Mater Studiorum Università di Bologna Muranty et al. BMC Plant Biology (2020) 20:2 https://doi.org/10.1186/s12870-019-2171-6 RESEARCH ARTICLE Open Access Using whole-genome SNP data to reconstruct a large multi-generation pedigree in apple germplasm Hélène Muranty1*† , Caroline Denancé1†, Laurence Feugey1, Jean-Luc Crépin2, Yves Barbier2, Stefano Tartarini3, Matthew Ordidge4, Michela Troggio5, Marc Lateur6, Hilde Nybom7, Frantisek Paprstein8, François Laurens1 and Charles-Eric Durel1 Abstract Background: Apple (Malus x domestica Borkh.) is one of the most important fruit tree crops of temperate areas, with great economic and cultural value. Apple cultivars can be maintained for centuries in plant collections through grafting, and some are thought to date as far back as Roman times. Molecular markers provide a means to reconstruct pedigrees and thus shed light on the recent history of migration and trade of biological materials. The objective of the present study was to identify relationships within a set of over 1400 mostly old apple cultivars using whole-genome SNP data (~ 253 K SNPs) in order to reconstruct pedigrees. Results: Using simple exclusion tests, based on counting the number of Mendelian errors, more than one thousand parent-offspring relations and 295 complete parent-offspring families were identified. Additionally, a grandparent couple was identified for the missing parental side of 26 parent-offspring pairings. Among the 407 parent-offspring relations without a second identified parent, 327 could be oriented because one of the individuals was an offspring in a complete family or by using historical data on parentage or date of recording. -

An Old Rose: the Apple
This is a republication of an article which first appeared in the March/April 2002 issue of Garden Compass Magazine New apple varieties never quite Rosaceae, the rose family, is vast, complex and downright confusing at times. completely overshadow the old ones because, as with roses, a variety is new only until the next This complexity has no better exemplar than the prince of the rose family, Malus, better known as the variety comes along and takes its apple. The apple is older in cultivation than the rose. It presents all the extremes in color, size, fragrance place. and plant character of its rose cousin plus an important added benefit—flavor! One can find apples to suit nearly every taste and cultural demand. Without any special care, apples grow where no roses dare. Hardy varieties like the Pippins, Pearmains, Snow, Lady and Northern Spy have been grown successfully in many different climates across the U.S. With 8,000-plus varieties worldwide and with new ones introduced annually, apple collectors in most climates are like kids in a candy store. New, Favorite and Powerhouse Apples New introductions such as Honeycrisp, Cameo and Pink Lady are adapted to a wide range of climates and are beginning to be planted in large quantities. The rich flavors of old favorites like Spitzenburg and Golden Russet Each one is a unique eating experience that are always a pleasant surprise for satisfies a modern taste—crunchy firmness, plenty inexperienced tasters. of sweetness and tantalizing flavor. Old and antique apples distinguish These new varieties show promise in the themselves with unusual skin competition for the #1 spot in the world’s colors and lingering aftertastes produce sections and farmers’ markets. -

Integrated Fire Blight Management WSTFRC YR1 Report
Continuing Report YR 1 Project Title: Integrated Fire Blight Management PI: Tianna DuPont Co-PI (2): Ken Johnson Organization: WSU Extension Organization: Oregon State University Telephone: (509) 293-8758 Telephone: (541) 737-5249 x5248 Email: [email protected] Email:[email protected] Address: Tree Fruit Research and Extension Address: Dept. Botany & Plant Pathology Address 2: 1100 N Western Ave Address 2: 2082 Cordley Hall City/State/Zip: Wenatchee WA 98801 City/State/Zip: Corvallis, OR 97321-2902 Co-PI(3): Kerik Cox Co-PI (4): Kari Peter Organization: Cornell University Organization: Penn State University Telephone: (315) 787-2401 Telephone: 717-677-6116 Ext. 223 Email: [email protected] Email: [email protected] Address: Cornell AgriTech Address: Fruit Research & Extension Ctr Address 2: 630 West North Street Address 2: PO Box 330, 290 University City/State/Zip: Geneva, NY, 14456 City/State/Zip: Biglerville, PA 17307 Cooperators: WA: Sean Gilbert, Gilbert Orchards; Travis Schoenwald, Gebber Farms; Paul Stikama, Douglas Fruit; Doug Stockwell, Arrowhead. Total Project Request: Year 1: $78,979 Year 2: $77,323 Other funding sources Agency Name: Applications made to SCRI Amt. requested/awarded: $346,000 Budget 1 Organization Name: WSU Contract Administrator: Kim Rains/Kate Roberts Telephone: 509.663.8181/509.335.2885 Email address: [email protected]/[email protected] Item 2019 2020 Salaries $3,7341 $11,6501 Benefits $1,4212 $4,4332 Wages Benefits Equipment Supplies $14,3243 $1,0004 Travel $500 $1000 Miscellaneous Plot Fees $2,100 $2,100 Total $22,079 $20,183 Footnotes: 1Salaries for a scientific assistant one-month year 1 and 3 months year 2 (DuPont).