OCUFLOX (Ofloxacin Ophthalmic Solution) 0.3% Sterile
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Oral Presentations September 23Rd - Rooms 1,2 and 3
Oral Presentations September 23rd - Rooms 1,2 and 3 Presentation Date Abstract Authors Presenter´s name - Theme Title Code indicated by the author 18498 Thomas Smits; Femke Gresnigt; Thomas Smits Clinical Toxicology/drugs of PERFORMANCE OF AN IMMUNOASSAY Eric Franssen; Milly Attema-de abuse METHOD FOR GAMMA-HYDROXYBUTYRIC Jonge ACID (GHB) IN PATIENTS PRESENTED AT THE EMERGENCY DEPARTMENT, A PROSPECTIVE STUDY 18499 Thomas Smits; Femke Gresnigt; Thomas Smits Clinical Toxicology/drugs of DO WE NEED POINT-OF-CARE TESTING OF Milly Attema-de Jonge; Eric abuse GAMMA-HYDROXYBUTYRIC ACID (GHB) AT Fransse THE EMERGENCY DEPARTMENT? September 23 18730 Lilian H.J. Richter; Julia Menges; Lea Wagmann Clinical Toxicology/drugs of NEW PSYCHOACTIVE SUBSTANCES: Lea Wagmann; Simon D. Brandt; abuse METABOLIC FATE, ISOZYME-MAPPING, 13:30 - 14:45 Folker Westphal; Veit Flockerzi; AND PLASMA PROTEIN BINDING OF 5-APB- ROOM 1 Markus R. Meyer NBOME, 2C-B-FLY-NB2ETO5CL, AND 2C-B- FLY-NBOME 18985 Annelies Cannaert; Marie Annelies Cannaert Clinical Toxicology/drugs of HIDE AND SEEK: OVERCOMING THE Deventer; Melissa Fogarty; abuse MASKING EFFECT OF OPIOID Amanda L.A. Mohr; Christophe P. ANTAGONISTS IN ACTIVITY-BASED Stove SCREENING TESTS 18740 Souleiman El Balkhi ; Roland Souleiman El Balkhi Clinical Toxicology/drugs of METABOLIC INTERACTIONS BETWEEN Lawson; Franck Saint-Marcoux abuse OXYCODONE, BENZODIAZEPINES OR DESIGNER BENZODIAZEPINES PLAY AN IMPORTANT ROLE IN OXYCODONE INTOXICATIONS 19050 Brenda de Winter F de Velde; MN Brenda de Winter Anti-infective drugs POPULATION -

Swedres-Svarm 2019
2019 SWEDRES|SVARM Sales of antibiotics and occurrence of antibiotic resistance in Sweden 2 SWEDRES |SVARM 2019 A report on Swedish Antibiotic Sales and Resistance in Human Medicine (Swedres) and Swedish Veterinary Antibiotic Resistance Monitoring (Svarm) Published by: Public Health Agency of Sweden and National Veterinary Institute Editors: Olov Aspevall and Vendela Wiener, Public Health Agency of Sweden Oskar Nilsson and Märit Pringle, National Veterinary Institute Addresses: The Public Health Agency of Sweden Solna. SE-171 82 Solna, Sweden Östersund. Box 505, SE-831 26 Östersund, Sweden Phone: +46 (0) 10 205 20 00 Fax: +46 (0) 8 32 83 30 E-mail: [email protected] www.folkhalsomyndigheten.se National Veterinary Institute SE-751 89 Uppsala, Sweden Phone: +46 (0) 18 67 40 00 Fax: +46 (0) 18 30 91 62 E-mail: [email protected] www.sva.se Text, tables and figures may be cited and reprinted only with reference to this report. Images, photographs and illustrations are protected by copyright. Suggested citation: Swedres-Svarm 2019. Sales of antibiotics and occurrence of resistance in Sweden. Solna/Uppsala ISSN1650-6332 ISSN 1650-6332 Article no. 19088 This title and previous Swedres and Svarm reports are available for downloading at www.folkhalsomyndigheten.se/ Scan the QR code to open Swedres-Svarm 2019 as a pdf in publicerat-material/ or at www.sva.se/swedres-svarm/ your mobile device, for reading and sharing. Use the camera in you’re mobile device or download a free Layout: Dsign Grafisk Form, Helen Eriksson AB QR code reader such as i-nigma in the App Store for Apple Print: Taberg Media Group, Taberg 2020 devices or in Google Play. -

Penetration of 0.3% Ciprofloxacin, 0.3% Ofloxacin, and 0.5
Brazilian Journal of Medical and Biological Research (2017) 50(7): e5901, http://dx.doi.org/10.1590/1414-431X20175901 ISSN 1414-431X 1/6 Penetration of 0.3% ciprofloxacin, 0.3% ofloxacin, and 0.5% moxifloxacin into the cornea and aqueous humor of enucleated human eyes G.C.M. Silva1, V.A.P. Jabor2, P.S. Bonato2, E.Z. Martinez3 and S.J. Faria-e-Sousa1 1Departamento de Oftalmologia, Faculdade de Medicina de Ribeirão Preto, Universidade de São Paulo, Ribeirão Preto, SP, Brasil 2Departamento de Física e Química, Faculdade de Ciências Farmacêuticas de Ribeirão Preto, Universidade de São Paulo, Ribeirão Preto, SP, Brasil 3Departamento de Medicina Social, Faculdade de Medicina de Ribeirão Preto, Universidade de São Paulo, Ribeirão Preto, SP, Brasil Abstract We aimed to quantify the penetration of ciprofloxacin, ofloxacin, and moxifloxacin into the cornea and aqueous humor of cadaver eyes. A total of 60 enucleated eyes, not eligible for corneal transplantation, were divided into three groups and immersed in commercial solutions of 0.3% ciprofloxacin, 0.3% ofloxacin, or 0.5% moxifloxacin for 10 min. Whole corneas and samples of aqueous humor were then harvested and frozen, and drug concentrations analyzed by liquid chromatography tandem mass spectrometry. The mean corneal concentration of moxifloxacin was twice as high as ofloxacin, and the latter was twice as high as ciprofloxacin. The mean concentration of moxifloxacin in the aqueous humor was four times higher than the other antibiotics, and the mean concentrations of ciprofloxacin and ofloxacin were statistically similar. The amount of drug that penetrated the anterior chamber after a 10-min immersion was far below the safe limit of endothelial toxicity of each preparation. -

Aetna Formulary Exclusions Drug List
Covered and non-covered drugs Drugs not covered – and their covered alternatives 2020 Advanced Control Plan – Aetna Formulary Exclusions Drug List 05.03.525.1B (7/20) Below is a list of medications that will not be covered without a Key prior authorization for medical necessity. If you continue using one of these drugs without prior approval, you may be required UPPERCASE Brand-name medicine to pay the full cost. Ask your doctor to choose one of the generic lowercase italics Generic medicine or brand formulary options listed below. Preferred Options For Excluded Medications1 Excluded drug name(s) Preferred option(s) ABILIFY aripiprazole, clozapine, olanzapine, quetiapine, quetiapine ext-rel, risperidone, ziprasidone, VRAYLAR ABSORICA isotretinoin ACANYA adapalene, benzoyl peroxide, clindamycin gel (except NDC^ 68682046275), clindamycin solution, clindamycin-benzoyl peroxide, erythromycin solution, erythromycin-benzoyl peroxide, tretinoin, EPIDUO, ONEXTON, TAZORAC ACIPHEX, esomeprazole, lansoprazole, omeprazole, pantoprazole, DEXILANT ACIPHEX SPRINKLE ACTICLATE doxycycline hyclate capsule, doxycycline hyclate tablet (except doxycycline hyclate tablet 50 mg [NDC^ 72143021160 only], 75 mg, 150 mg), minocycline, tetracycline ACTOS pioglitazone ACUVAIL bromfenac, diclofenac, ketorolac, PROLENSA acyclovir cream acyclovir (except acyclovir cream), valacyclovir ADCIRCA sildenafil, tadalafil ADZENYS XR-ODT amphetamine-dextroamphetamine mixed salts ext-rel†, dexmethylphenidate ext-rel, dextroamphetamine ext-rel, methylphenidate ext-rel†, MYDAYIS, -

To Hold (Enteral Feeding) Or Not to Hold: That IS the Question; a Commentary and Tutorial
NUTRITIONINFLAMMATORY ISSUES BOWEL IN GASTROENTEROLOGY, DISEASE: A PRACTICAL SERIES APPROACH, #101 SERIES #73 Carol Rees Parrish, M.S., R.D., Series Editor To Hold (Enteral Feeding) or Not to Hold: That IS the Question; A Commentary and Tutorial by Lingtak-Neander Chan, PharmD, BCNSP Enteral nutrition may interfere with drug absorption and lead to therapeutic failure. The best management plan to minimize this interaction remains controversial. One of the solutions to this clinical issue involves withholding enteral feeding for a period of time in order to minimize the potential risk factors that interfere with oral bioavailability of drugs. Although data from the literature suggest that this approach is associated with limited success in some cases, the length of time enteral feeding was held varied among studies. This article serves as a tutorial guide to help clinicians determine when these concerns may be more clinically significant and what actions can be considered to optimize patient outcomes. INTRODUCTION hould enteral nutrition (EN) be withheld before the new feeding regimen by the patient may become this medication is administered? What do we an issue. From the pharmacotherapeutic perspective, actually accomplish by holding EN? How long there are concerns that the absorption pattern and Sshould EN be withheld? Should EN be stopped both bioavailability of drug are altered by EN, thus affecting before and after drug administration? These are some of the safety and efficacy profiles of medications. The aim the questions that many clinicians have been wondering of this article is to discuss when these concerns are about for a long time. Often times, the responses can clinically significant and what actions could be taken be quite different, and even conflicting, depending to optimize patient care. -

Danmap 2006.Pmd
DANMAP 2006 DANMAP 2006 DANMAP 2006 - Use of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from food animals, foods and humans in Denmark Statens Serum Institut Danish Veterinary and Food Administration Danish Medicines Agency National Veterinary Institute, Technical University of Denmark National Food Institute, Technical University of Denmark Editors: Hanne-Dorthe Emborg Danish Zoonosis Centre National Food Institute, Technical University of Denmark Mørkhøj Bygade 19 Contents DK - 2860 Søborg Anette M. Hammerum National Center for Antimicrobials and Contributors to the 2006 Infection Control DANMAP Report 4 Statens Serum Institut Artillerivej 5 DK - 2300 Copenhagen Introduction 6 DANMAP board: National Food Institute, Acknowledgements 6 Technical University of Denmark: Ole E. Heuer Frank Aarestrup List of abbreviations 7 National Veterinary Institute, Tecnical University of Denmark: Sammendrag 9 Flemming Bager Danish Veterinary and Food Administration: Summary 12 Justin C. Ajufo Annette Cleveland Nielsen Statens Serum Institut: Demographic data 15 Dominique L. Monnet Niels Frimodt-Møller Anette M. Hammerum Antimicrobial consumption 17 Danish Medicines Agency: Consumption in animals 17 Jan Poulsen Consumption in humans 24 Layout: Susanne Carlsson Danish Zoonosis Centre Resistance in zoonotic bacteria 33 Printing: Schultz Grafisk A/S DANMAP 2006 - September 2007 Salmonella 33 ISSN 1600-2032 Campylobacter 43 Text and tables may be cited and reprinted only with reference to this report. Resistance in indicator bacteria 47 Reprints can be ordered from: Enterococci 47 National Food Institute Escherichia coli 58 Danish Zoonosis Centre Tecnical University of Denmark Mørkhøj Bygade 19 DK - 2860 Søborg Resistance in bacteria from Phone: +45 7234 - 7084 diagnostic submissions 65 Fax: +45 7234 - 7028 E. -

LEVAQUIN (Levofloxacin) Tablets Are Supplied As 250, 500, and 750 Mg Capsule-Shaped, Coated Tablets
LEVAQUIN (levofloxacin) TABLETS LEVAQUIN (levofloxacin) ORAL SOLUTION LEVAQUIN (levofloxacin) INJECTION LEVAQUIN (levofloxacin in 5% dextrose) INJECTION To reduce the development of drug-resistant bacteria and maintain the effectiveness of LEVAQUIN (levofloxacin) and other antibacterial drugs, LEVAQUIN should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria. DESCRIPTION LEVAQUIN (levofloxacin) is a synthetic broad spectrum antibacterial agent for oral and intravenous administration. Chemically, levofloxacin, a chiral fluorinated carboxyquinolone, is the pure (-)-(S)-enantiomer of the racemic drug substance ofloxacin. The chemical name is (-)-(S)-9-fluoro-2,3-dihydro-3-methyl-10-(4-methyl- 1-piperazinyl)-7-oxo-7H-pyrido[1,2,3-de]-1,4-benzoxazine-6-carboxylic acid hemihydrate. O F COOH 1/2 H2O N N N O CH3 H3C The chemical structure is: H Its empirical formula is C18H20FN3O4 • ½ H2O and its molecular weight is 370.38. Levofloxacin is a light yellowish-white to yellow-white crystal or crystalline powder. The molecule exists as a zwitterion at the pH conditions in the small intestine. The data demonstrate that from pH 0.6 to 5.8, the solubility of levofloxacin is essentially constant (approximately 100 mg/mL). Levofloxacin is considered soluble to freely soluble in this pH range, as defined by USP nomenclature. Above pH 5.8, the solubility increases rapidly to its maximum at pH 6.7 (272 mg/mL) and is considered freely soluble in this range. Above pH 6.7, the solubility decreases and reaches a minimum value (about 50 mg/mL) at a pH of approximately 6.9. -

BMJ Open Is Committed to Open Peer Review. As Part of This Commitment We Make the Peer Review History of Every Article We Publish Publicly Available
BMJ Open is committed to open peer review. As part of this commitment we make the peer review history of every article we publish publicly available. When an article is published we post the peer reviewers’ comments and the authors’ responses online. We also post the versions of the paper that were used during peer review. These are the versions that the peer review comments apply to. The versions of the paper that follow are the versions that were submitted during the peer review process. They are not the versions of record or the final published versions. They should not be cited or distributed as the published version of this manuscript. BMJ Open is an open access journal and the full, final, typeset and author-corrected version of record of the manuscript is available on our site with no access controls, subscription charges or pay-per-view fees (http://bmjopen.bmj.com). If you have any questions on BMJ Open’s open peer review process please email [email protected] BMJ Open Pediatric drug utilization in the Western Pacific region: Australia, Japan, South Korea, Hong Kong and Taiwan Journal: BMJ Open ManuscriptFor ID peerbmjopen-2019-032426 review only Article Type: Research Date Submitted by the 27-Jun-2019 Author: Complete List of Authors: Brauer, Ruth; University College London, Research Department of Practice and Policy, School of Pharmacy Wong, Ian; University College London, Research Department of Practice and Policy, School of Pharmacy; University of Hong Kong, Centre for Safe Medication Practice and Research, Department -

Trend Analysis of Antibiotics Consumption Using WHO Aware Classification in Tertiary Care Hospital
International Journal of Basic & Clinical Pharmacology Bhardwaj A et al. Int J Basic Clin Pharmacol. 2020 Nov;9(11):1675-1680 http:// www.ijbcp.com pISSN 2319-2003 | eISSN 2279-0780 DOI: https://dx.doi.org/10.18203/2319-2003.ijbcp20204493 Original Research Article Trend analysis of antibiotics consumption using WHO AWaRe classification in tertiary care hospital Ankit Bhardwaj*, Kaveri Kapoor, Vivek Singh Saraswathi institute of Medical Sciences, Pilakhuwa, Hapur, Uttar Pradesh, India Received: 21 August 2020 Accepted: 21 September 2020 *Correspondence: Dr. Ankit Bhardwaj, Email: [email protected] Copyright: © the author(s), publisher and licensee Medip Academy. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Background: Aim of the study was to assess trend in antibiotics consumption pattern from 2016 to 2019 using AWaRe classification, ATC and Defined daily dose methodology (DDD) in a tertiary care hospital. Antibiotics are crucial for treating infectious diseases and have significantly improved the prognosis of patients with infectious diseases, reducing morbidity and mortality. The aim of the study is to classify the antibiotic based on WHO AWaRe classification and compare their four-year consumption trends. The study was conducted at a tertiary care center, Pilakhuwa, Hapur. Antibiotic procurement data for a period of 4 years (2016-2019) was collected from the Central medical store. Methods: This is a retrospective time series analysis of systemic antibiotics with no intervention at patient level. Antibiotic procurement was taken as proxy for consumption assuming that same has been used. -

FLOXIN Tablets (Ofloxacin Tablets)
FLOXIN® Tablets (Ofloxacin Tablets) WARNING: Fluoroquinolones, including FLOXIN®, are associated with an increased risk of tendinitis and tendon rupture in all ages. This risk is further increased in older patients usually over 60 years of age, in patients taking corticosteroid drugs, and in patients with kidney, heart or lung transplants (See WARNINGS). To reduce the development of drug-resistant bacteria and maintain the effectiveness of FLOXIN® (ofloxacin tablets) Tablets and other antibacterial drugs, FLOXIN® (ofloxacin tablets) Tablets should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria. DESCRIPTION FLOXIN® (ofloxacin tablets) Tablets is a synthetic broad-spectrum antimicrobial agent for oral administration. Chemically, ofloxacin, a fluorinated carboxyquinolone, is the racemate, (±)-9-fluoro-2,3-dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-7 oxo-7H-pyrido[1,2,3-de]-1,4-benzoxazine-6-carboxylic acid. The chemical structure is: Its empirical formula is C18H20FN3O4, and its molecular weight is 361.4 Ofloxacin is an off-white to pale yellow crystalline powder. The molecule exists as a zwitterion at the pH conditions in the small intestine. The relative solubility characteristics of ofloxacin at room temperature, as defined by USP nomenclature, indicate that ofloxacin is considered to be soluble in aqueous solutions with pH between 2 and 5. It is sparingly to slightly soluble in aqueous solutions with pH 7 (solubility falls to 4 mg/mL) and freely soluble in aqueous solutions with pH above 9. Ofloxacin has the potential to form stable coordination compounds with many metal ions. This in vitro chelation potential has the following formation order: Fe+3 > Al+3 > Cu +2 > Ni+2 > Pb+2 > Zn+2 > Mg+2 > Ca+2 > Ba+2. -
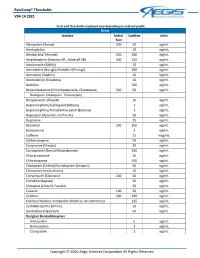
Paincomp® Thresholds V04.14.2021 Copyright © 2020 Aegis Sciences
PainComp® Thresholds V04.14.2021 Tests and thresholds employed vary depending on ordered profile Urine Analyte Initial Confirm Units Test Alprazolam (Xanax) 200 50 ng/mL Amitriptyline 10 ng/mL Amobarbital (Amytal) 200 200 ng/mL Amphetamine (Adzenys ER , Adderall XR) 500 250 ng/mL Aripiprazole (Abilify) 10 ng/mL Armodafinil (Nuvigil)/Modafinil (Provigil) 100 ng/mL Asenapine (Saphris) 10 ng/mL Atomoxetine (Strattera) 10 ng/mL Baclofen 500 ng/mL Benzodiazepines (Chlordiazepoxide, Clorazepate, 200 50 ng/mL Diazepam, Oxazepam, Temazepam) Brexpiprazole (Rexulti) 10 ng/mL Buprenorphine/Sublingual (Belbuca) 1 ng/mL Buprenorphine/Transdermal patch (Butrans) 1 ng/mL Bupropion (Aplenzin, Forfivo XL) 50 ng/mL Buspirone 25 ng/mL Butalbital 200 200 ng/mL Butorphanol 1 ng/mL Caffeine 15 mcg/mL Carbamazepine 50 ng/mL Cariprazine (Vraylar) 20 ng/mL Carisoprodol (Soma)/Meprobamate 200 ng/mL Chlorpromazine 10 ng/mL Chlorzoxazone 500 ng/mL Citalopram (Celexa)/Escitalopram (Lexapro) 50 ng/mL Clomipramine (Anafranil) 10 ng/mL Clonazepam (Klonopin) 200 50 ng/mL Clonidine (Kapvay) 50 ng/mL Clozapine (Clozaril, Fazaclo) 50 ng/mL Cocaine 100 50 ng/mL Codeine 200 100 ng/mL Cotinine/Nicotine metabolite (Habitrol, Nicoderm CQ) 125 ng/mL Cyclobenzaprine (Amrix) 10 ng/mL Dantrolene (Dantrium) 50 ng/mL Designer Benzodiazepines Adinazolam 5 ng/mL Bromazolam 1 ng/mL Clonazolam 1 ng/mL Copyright © 2020 Aegis Sciences Corporation All Rights Reserved PainComp® Thresholds V04.14.2021 Deschloroetizolam 1 ng/mL Diclazepam 1 ng/mL Etizolam 1 ng/mL Flualprazolam 1 ng/mL Flubromazepam -

Alcohol and Other Drug Withdrawal: Practice Guidelines 2018 Were Funded by the Victorian Department of Health and Human Services
ALCOHOL AND DRUG ALCOHOL AND OTHER DRUG WITHDRAWAL GUIDELINES THIRD EDITION 2018 Dr Victoria Manning Dr Shalini Arunogiri Dr Matthew Frei Dr Kelly Ridley Katherine Mroz Sam Campbell Prof Dan Lubman ALCOHOL AND OTHER DRUG WITHDRAWAL GUIDELINES Turning Point ACKNOWLEDGEMENTS In the revisions of this resource Turning Point acknowledges the considerable contribution of the following clinicians: Dr Keri Alexander – Turning Point (Eastern Health) Mr Teddy Sikhali – Turning Point (Eastern Health) Ms Rose McCrohan – Uniting ReGen Dr Benny Monheit – Southcity Clinic/Alfred Hospital Members of the Victorian Non-Residential Withdrawal Nurses network. Authors of the previous versions: Pauline Kenny, Amy Swan, Lynda Berends, Linda Jenner, Barbara Hunter & Janette Mugavin. We also acknowledge the contribution of members of the Victorian AoD sector on the previous version of the guidelines which included; Moses Abbatangelo, Regina Brindle, Dr Malcolm Dobbin, Mal Dorian, Dr Matthew Frei, Deb Little Salvation Army, Dr Benny Monheit, Donna Ribton-Turner, Glenn Rutter. Copyright © 2018 State of Victoria 3rd edition printed 2018. ISBN: 978-1-74001-019-1 Paper ISBN: 978-1-74001-020-7 epub The withdrawal guidelines were funded by the Victorian Government. Copyright enquiries can be made to Turning Point, 110 Church Street, Richmond, Victoria 3121, Australia. Published by Turning Point. The Alcohol and Other Drug Withdrawal: Practice Guidelines 2018 were funded by the Victorian Department of Health and Human Services. The responsibility for all statements made in this document lie with the authors. The views of the authors do not necessarily reflect the views and position of the Department of Health and Human Services or Turning Point.