Black Willow (Salix Nigra) Use in Phytoremediation Techniques To
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

State of New York City's Plants 2018
STATE OF NEW YORK CITY’S PLANTS 2018 Daniel Atha & Brian Boom © 2018 The New York Botanical Garden All rights reserved ISBN 978-0-89327-955-4 Center for Conservation Strategy The New York Botanical Garden 2900 Southern Boulevard Bronx, NY 10458 All photos NYBG staff Citation: Atha, D. and B. Boom. 2018. State of New York City’s Plants 2018. Center for Conservation Strategy. The New York Botanical Garden, Bronx, NY. 132 pp. STATE OF NEW YORK CITY’S PLANTS 2018 4 EXECUTIVE SUMMARY 6 INTRODUCTION 10 DOCUMENTING THE CITY’S PLANTS 10 The Flora of New York City 11 Rare Species 14 Focus on Specific Area 16 Botanical Spectacle: Summer Snow 18 CITIZEN SCIENCE 20 THREATS TO THE CITY’S PLANTS 24 NEW YORK STATE PROHIBITED AND REGULATED INVASIVE SPECIES FOUND IN NEW YORK CITY 26 LOOKING AHEAD 27 CONTRIBUTORS AND ACKNOWLEGMENTS 30 LITERATURE CITED 31 APPENDIX Checklist of the Spontaneous Vascular Plants of New York City 32 Ferns and Fern Allies 35 Gymnosperms 36 Nymphaeales and Magnoliids 37 Monocots 67 Dicots 3 EXECUTIVE SUMMARY This report, State of New York City’s Plants 2018, is the first rankings of rare, threatened, endangered, and extinct species of what is envisioned by the Center for Conservation Strategy known from New York City, and based on this compilation of The New York Botanical Garden as annual updates thirteen percent of the City’s flora is imperiled or extinct in New summarizing the status of the spontaneous plant species of the York City. five boroughs of New York City. This year’s report deals with the City’s vascular plants (ferns and fern allies, gymnosperms, We have begun the process of assessing conservation status and flowering plants), but in the future it is planned to phase in at the local level for all species. -

Short Rotation Intensive Culture of Willow, Spent Mushroom Substrate
plants Article Short Rotation Intensive Culture of Willow, Spent Mushroom Substrate and Ramial Chipped Wood for Bioremediation of a Contaminated Site Used for Land Farming Activities of a Former Petrochemical Plant Maxime Fortin Faubert 1 , Mohamed Hijri 1,2 and Michel Labrecque 1,* 1 Institut de Recherche en biologie végétale, Université de Montréal and Jardin Botanique de Montréal, 4101 Sherbrooke East, Montréal, QC H1X 2B2, Canada; [email protected] (M.F.F.); [email protected] (M.H.) 2 African Genome Center, Mohammed VI Polytechnic University (UM6P), Lot 660, Hay Moulay Rachid, Ben Guerir 43150, Morocco * Correspondence: [email protected]; Tel.: +1-514-978-1862 Abstract: The aim of this study was to investigate the bioremediation impacts of willows grown in short rotation intensive culture (SRIC) and supplemented or not with spent mushroom substrate (SMS) and ramial chipped wood (RCW). Results did not show that SMS significantly improved either biomass production or phytoremediation efficiency. After the three growing seasons, RCW- amended S. miyabeana accumulated significantly more Zn in the shoots, and greater increases of some PAHs were found in the soil of RCW-amended plots than in the soil of the two other ground Citation: Fortin Faubert, M.; Hijri, cover treatments’ plots. Significantly higher Cd concentrations were found in the shoots of cultivar M.; Labrecque, M. Short Rotation ‘SX61’. The results suggest that ‘SX61’ have reduced the natural attenuation of C10-C50 that occurred Intensive Culture of Willow, Spent in the unvegetated control plots. The presence of willows also tended to increase the total soil Mushroom Substrate and Ramial concentrations of PCBs. -

The Use of Vegetation in the Stabilization, Reclamation, and Remediation of Impacted INDOT Soils
Final Report FHWA/IN/JTRP-2004/17 The Use of Vegetation in the Stabilization, Reclamation, and Remediation of Impacted INDOT Soils by Mark S. McClain Graduate Research Assistant and M. Katherine Banks Professor School of Civil Engineering and A. Paul Schwab Department of Agronomy Purdue University Joint Transportation Research Program Project No. C-36-68T File No. 4-7-20 SPR-2624 Prepared in Cooperation with the Indiana Department of Transportation and the U.S. Department of Transportation Federal Highway Administration The contents of this report reflect the views of the author who is responsible for the facts and the accuracy of the data presented herein. The contents do not necessarily reflect the official views or policies of the Indiana Department of Transportation or the Fedeeral Highway Administration at the time of publication. This report does not constitute a standard, specification, or regulation. Purdue University West Lafayette, Indiana 47907 October 2004 TECHNICAL Summary Technology Transfer and Project Implementation Information INDOT Research TRB Subject Code: 23-8 Ecological Abatement October 2004 Publication No.: FHWA/IN/JTRP-2004/17, SPR-2624 Final Report The Use of Vegetation in the Stabilization, Reclamation, and Remediation of Impacted INDOT Soils Introduction Soils can be severely impacted by transportation- Phytoremediation uses plants to degrade, extract, related activities including highway construction, contain, or immobilize contaminants from soil and renovations, maintenance, and accidental spills. water. Phytoremediation is an innovative, cost- Reclamation or remediation of soils contaminated effective alternative to more conventional treatment by salts, solvents, paints, petroleum, and metals methods used in the remediation of hazardous may be necessary to comply with current waste sites. -

Phytoremediation Potential of Fast-Growing Energy Plants: Challenges and Perspectives – a Review
Pol. J. Environ. Stud. Vol. 29, No. 1 (2020), 505-516 DOI: 10.15244/pjoes/101621 ONLINE PUBLICATION DATE: 2019-09-10 Review Phytoremediation Potential of Fast-Growing Energy Plants: Challenges and Perspectives – a Review Martin Hauptvogl1*, Marián Kotrla2, Martin Prčík1, Žaneta Pauková3, Marián Kováčik4, Tomáš Lošák5 1Department of Environmental Management, Slovak University of Agriculture in Nitra, Slovakia 2Department of Regional Bioenergetics, Slovak University of Agriculture in Nitra, Slovakia 3Department of Regional Bioenergetics , Slovak University of Agriculture in Nitra, Slovakia 4Department of EU Policies, Slovak University of Agriculture in Nitra, Slovakia 5Department of Environmentalistics and Natural Resources, Mendel University in Brno, Czech Republic Received: 23 July 2018 Accepted: 15 December 2018 Abstract Contamination of soil by toxic elements is a global issue of growing importance due to the increased anthropogenic impact on the natural environment. Conventional methods of soil decontamination possess disadvantages in forms of environmental and financial burdens. This fact leads to the search for alternative approaches of remediation of contaminated sites. One such approach includes phytoremediation. Phytoremediation advantages consist of low costs and small environmental impact. Several fast-growing energy plant species are suitable for phytoremediation purposes. Our article focuses on the phytoremediation potential of energy woody crops of Salix and Populus, and energy grasses Miscanthus and Arundo, which are grown primarily for biomass production. This approach links the environmentally friendly and economically less demanding remediation approach with the production of the local sustainable form of energy that decreases dependency on external energy supplies. Energy plants are able to provide high biomass yields in a short period of time, they are resistant against abiotic stress conditions and have the ability to accumulate toxic substances, thus helping to restore the desirable soil properties. -
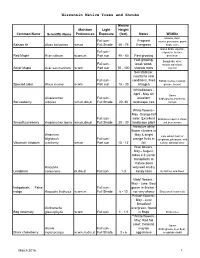
Wisconsin Native Trees and Shrubs
Wisconsin Native Trees and Shrubs Mature Moisture Light Height Common Name Scientific Name Preferences Exposure (feet) Notes Wildlife Grouse, deer, Full sun - Fragrant moose,porcupine, game Balsam fir Abies balsamea wm,m Full Shade 40 - 75 Evergreen birds, mice Game birds, squirrel, Full sun - chipmunk, beaver, Red Maple Acer rubrum w,wm,m Part sun 40 - 60 Fast growing deer,bear Fast growing, Songbirds, deer, Full sun - weak wood, racoon,waterfowl, Silver Maple Acer saccharinum w,wm Part sun 75 - 100 shallow roots squirrel Soil stablizer, neutral to acid Full sun - conditions, fixes Rabbit,moose,muskrat, Specled alder Alnus incana w,wm Part sun 15 - 30 nitrogen grouse, beaver Whiteflowers - April - May An Game Amelanchier Full sun - excellent birds,grouse,skunk,fox, Serviceberry arborea wm,m,dm,d Full Shade 20 -30 landscape tree racoon White flowers - May Orange fall Full sun - color Excellent Birds,bear,squirrel,chipm Smooth juneberry Amelanchier laevis wm,m,dm,d Full Shade 20 - 30 landscape plant unk,deer,moose Attractive white flower clusters in American May & bright Late winter food for Highbush Full sun - orange fruits in songbirds, pheasant, wild Viburnum trilobum cranberry wm,m Part sun 10 - 13' fall turkey, whitetail deer Blue flowers, May - August; takes 2-3 yrs for transplants to mature;does Amorpha very well on dry Leadplant canescens m,dm,d Full sun 1-3 sandy sites Butterflies and Bees Violet flowers - May - June Best Indigobush; False Full sun - grown in thicket - indigo Amorpha fruticosa w,wm,m Full Shade 6 - 12 not very -

Interest Increases in Using Plants for Environmental Remediation
Specialty Plant Products Interest Increases in Using Plants For Environmental Remediation In an effort to meet environmental regulations of the last three decades, environmental remediation has developed into a multibillion dollar industry. The high cost of many traditional methods is causing many organizations to look to lower cost alternatives. Bioremediation is a commercial remediation technology with a growing market and continuing research. Phytoremediation is another potential low-cost technology that is currently being investigated for many remediation applications. Health and environmental risks of pollution have become Table 14--Soil remediation technologies more apparent throughout the world over the past several Method In situ Ex situ decades. Air, water, and soil contaminants can include nu- merous organic and inorganic substances, such as munici- Physical Soil vapor extraction Landfilling pal waste and sewage, various gaseous emissions, fertiliz- Thermally enhanced Incineration ers, pesticides, chemicals, heavy metals, and radionuclides soil vapor extraction Thermal desorption (radioactive substances). Contaminants can cause land and Containment systems Soil vapor extraction groundwater to be unusable. In addition, animals and in- and barriers sects may come in contact with a contaminant, thus intro- ducing a toxic substance into the food chain. Because of in- Chemical Soil flushing Soil washing creased public awareness and concern, environmental Solidification Solidification regulations have been created to not only prevent -
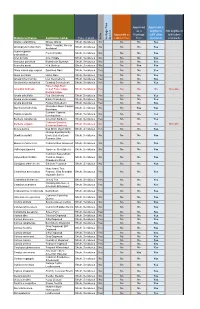
Botanical Name Common Name
Approved Approved & as a eligible to Not eligible to Approved as Frontage fulfill other fulfill other Type of plant a Street Tree Tree standards standards Heritage Tree Tree Heritage Species Botanical Name Common name Native Abelia x grandiflora Glossy Abelia Shrub, Deciduous No No No Yes White Forsytha; Korean Abeliophyllum distichum Shrub, Deciduous No No No Yes Abelialeaf Acanthropanax Fiveleaf Aralia Shrub, Deciduous No No No Yes sieboldianus Acer ginnala Amur Maple Shrub, Deciduous No No No Yes Aesculus parviflora Bottlebrush Buckeye Shrub, Deciduous No No No Yes Aesculus pavia Red Buckeye Shrub, Deciduous No No Yes Yes Alnus incana ssp. rugosa Speckled Alder Shrub, Deciduous Yes No No Yes Alnus serrulata Hazel Alder Shrub, Deciduous Yes No No Yes Amelanchier humilis Low Serviceberry Shrub, Deciduous Yes No No Yes Amelanchier stolonifera Running Serviceberry Shrub, Deciduous Yes No No Yes False Indigo Bush; Amorpha fruticosa Desert False Indigo; Shrub, Deciduous Yes No No No Not eligible Bastard Indigo Aronia arbutifolia Red Chokeberry Shrub, Deciduous Yes No No Yes Aronia melanocarpa Black Chokeberry Shrub, Deciduous Yes No No Yes Aronia prunifolia Purple Chokeberry Shrub, Deciduous Yes No No Yes Groundsel-Bush; Eastern Baccharis halimifolia Shrub, Deciduous No No Yes Yes Baccharis Summer Cypress; Bassia scoparia Shrub, Deciduous No No No Yes Burning-Bush Berberis canadensis American Barberry Shrub, Deciduous Yes No No Yes Common Barberry; Berberis vulgaris Shrub, Deciduous No No No No Not eligible European Barberry Betula pumila -

Phytoremediation of Wood Preservatives FWRC
Phytoremediation of Wood Preservatives FWRC Soil and waste contaminated with wood for contaminated areas: 1) enhanced microbial preservatives have been found in many former degradation of contaminants within the rhizosphere, and active wood treating plants, resulting from 2) hyperaccumulators where plants uptake and store past practices and accidental spillage. During the harmful contaminants, commonly heavy metals, in spring of 2001, newspaper articles in Florida raised their roots and shoots, 3) rhizofiltration where plant a public alarm over the perception that arsenate roots absorb, concentrate, or precipitate heavy metal from CCA (chromated copper arsenate) treated ions from water and, 4) phytovolatilization where wood—used in playground equipment— was leaching plants uptake volatile organic compounds (VOCs) in into soils and potentially into groundwater reserves. groundwater allowing them to be released into the As a result, CCA treated wood was voluntarily atmosphere via the stomata openings. taken off the market by the industry. Physical Surface waters and shallow aquifers were the removal of contaminated soils followed by treatment first sites where plants were applied as a method of technologies—soil washing, solidification cleanup. Many different plants and trees can and stabilization, chemical remove or degrade toxic pollutants. treatment,vitrification, thermal The primary disadvantage in using desorption, electrokinetics, trees for environmental cleanup and incineration—is not a is the time needed for tree cost effective soil cleanup growth. Faster growing method for heavy metal flora like grasses may be contaminated water. The suited for some situations. most common technologies Although the roots of include: coagulation/ grasses do not penetrate filtration (activated carbon), the soil at the same depths lime softening, chemical as tree roots, they can treatment, reverse osmosis, proliferate within the topsoil. -

Responses of Black Willow (Salix Nigra) Cuttings to Simulated Herbivory and flooding
Acta Oecologica 28 (2005) 173–180 www.elsevier.com/locate/actoec Original article Responses of black willow (Salix nigra) cuttings to simulated herbivory and flooding Shuwen Li a,*, Lili T. Martin a, S. Reza Pezeshki a, F. Douglas Shields Jr. b a Department of Biology, The University of Memphis, Memphis, TN 38152, USA b USDA-ARS National Sedimentation Laboratory, P.O. Box 1157, Oxford, MS 38655, USA Received 7 January 2004; accepted 31 March 2005 Available online 04 May 2005 Abstract Herbivory and flooding influence plant species composition and diversity in many wetland ecosystems. Black willow (Salix nigra) natu- rally occurs in floodplains and riparian zones of the southeastern United States. Cuttings from this species are used as a bioengineering tool for streambank stabilization and habitat rehabilitation. The present study was conducted to evaluate the photosynthetic and growth responses of black willow to simulated herbivory and flooding. Potted cuttings were subjected to three levels of single-event herbivory: no herbivory (control), light herbivory, and heavy herbivory; and three levels of flooding conditions: no flooding (control), continuous flooding, and peri- odic flooding. Results indicated that elevated stomatal conductance partially contributed to the increased net photosynthesis noted under both levels of herbivory on day 30. However, chlorophyll content was not responsible for the observed compensatory photosynthesis. Cuttings subjected to heavy herbivory accumulated the lowest biomass even though they had the highest height growth by the conclusion of the experiment. In addition, a reduction in root/shoot ratio was noted for plants subjected to continuous flooding with no herbivory. However, continuously flooded, lightly clipped plants allocated more resources to roots than shoots. -

Sustainable Use of Plants for Heavy Metal Removal from Water: Phytoremediation
A.P. Bhat and P.P. Bhat (2016) Int J Appl Sci Biotechnol, Vol 4(2): 150-154 DOI: 10.3126/ijasbt.v4i2.14742 Mini Review SUSTAINABLE USE OF PLANTS FOR HEAVY METAL REMOVAL FROM WATER: PHYTOREMEDIATION Akash P. Bhat1* and Pooja P. Bhat2 1Department of Chemical Engineering, Thadomal Shahani Engineering College, Bandra West, Mumbai 400050, Maharashtra, India, 2Department of Botany, Ramnarain Ruia College, Matunga, Mumbai 400019, Maharashtra, India. *Corresponding Author email: [email protected] Abstract There are various methods for removal of heavy metals from contaminated water and many of them can be costly and also consume a lot of resources. Phytoremediation is the use of plants as a filter for removal of unwanted elements and substances from contaminated water. This process is called rhizofiltration. Phytoremediation has not achieved a lot of importance on large scale level. This review- study shows how several species like Brassica juncea and Chenopodium amaranticolor, Pistia stratiotes, Helianthus annuus L. and Phaseolus vulgaris L. var. vulgaris, Eleocharis acicularis, Lemna minor L., Phragmites australis and Eichhornia Crassipes can be used for effective removal of heavy metals. These species are selected based on a review on various studies on rhizofiltration. Hence rhizofiltration can be an eco-friendly and innovative method of removal of heavy metals and has to be applied for large scale treatment of heavy metals in real time waters. Keywords: Phytoremediation; Rhizofiltration; Plants; Heavy metals; Removal Introduction processes should be at the utmost important place for Heavy metals and pollutants in water and aqueous wastes combating global warming and pollution. are a huge threat currently to the environment. -

Salix Nigra Marsh
Salix nigra Marsh Salix nigra Marsh. Black Willow Salicaceae -- Willow family J. A. Pitcher and J. S. McKnight Black willow (Salix nigra) is the largest and the only commercially important willow of about 90 species native to North America. It is more distinctly a tree throughout its range than any other native willow; 27 species attain tree size in only part of their range (3). Other names sometimes used are swamp willow, Goodding willow, southwestern black willow, Dudley willow, and sauz (Spanish). This short-lived, fast-growing tree reaches its maximum size and development in the lower Mississippi River Valley and bottom lands of the Gulf Coastal Plain (4). Stringent requirements of seed germination and seedling establishment limit black willow to wet soils near water courses (5), especially floodplains, where it often grows in pure stands. Black willow is used for a variety of wooden products and the tree, with its dense root system, is excellent for stabilizing eroding lands. Habitat Native Range Black willow is found throughout the Eastern United States and adjacent parts of Canada and Mexico. The range extends from southern New Brunswick and central Maine west in Quebec, southern Ontario, and central Michigan to southeastern Minnesota; south and west to the Rio Grande just below its confluence with the Pecos River; and east along the gulf coast, through the Florida panhandle and southern Georgia. Some authorities consider Salix gooddingii as a variety of S. nigra, which extends the range to the Western United States (3,9). http://www.na.fs.fed.us/spfo/pubs/silvics_manual/volume_2/salix/nigra.htm (1 of 9)1/4/2009 4:08:21 PM Salix nigra Marsh -The native range of black willow. -

THE ABIOTIC STRESS RESPONSE of HYDROPONIC VETIVER GRASS (Chrysopogon Zizanioides L.) to ACID MINE DRAINAGE and ITS POTENTIAL for ENVIRONMENTAL REMEDIATION
Michigan Technological University Digital Commons @ Michigan Tech Dissertations, Master's Theses and Master's Reports 2018 THE ABIOTIC STRESS RESPONSE OF HYDROPONIC VETIVER GRASS (Chrysopogon zizanioides L.) TO ACID MINE DRAINAGE AND ITS POTENTIAL FOR ENVIRONMENTAL REMEDIATION Jef Kiiskila Michigan Technological University, [email protected] Copyright 2018 Jef Kiiskila Recommended Citation Kiiskila, Jef, "THE ABIOTIC STRESS RESPONSE OF HYDROPONIC VETIVER GRASS (Chrysopogon zizanioides L.) TO ACID MINE DRAINAGE AND ITS POTENTIAL FOR ENVIRONMENTAL REMEDIATION", Open Access Dissertation, Michigan Technological University, 2018. https://doi.org/10.37099/mtu.dc.etdr/763 Follow this and additional works at: https://digitalcommons.mtu.edu/etdr Part of the Biochemistry Commons, Environmental Sciences Commons, and the Plant Biology Commons THE ABIOTIC STRESS RESPONSE OF HYDROPONIC VETIVER GRASS (Chrysopogon zizanioides L.) TO ACID MINE DRAINAGE AND ITS POTENTIAL FOR ENVIRONMENTAL REMEDIATION By Jeffrey D. Kiiskila A DISSERTATION Submitted in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY In Biochemistry and Molecular Biology MICHIGAN TECHNOLOGICAL UNIVERSITY 2018 © 2018 Jeffrey D. Kiiskila This dissertation has been approved in partial fulfillment of the requirements for the Degree of DOCTOR OF PHILOSOPHY in Biochemistry and Molecular Biology. Department of Biological Sciences Dissertation Advisor: Rupali Datta Committee Member: Dibyendu Sarkar Committee Member: Tarun K. Dam Committee Member: Victor Busov Department