Non-Commercial Use Only
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A New Approach for Purification of Recombinant
Biotechnology & Biotechnological Equipment ISSN: 1310-2818 (Print) 1314-3530 (Online) Journal homepage: https://www.tandfonline.com/loi/tbeq20 Phytoplankton Based Assessment of the Ecological Status and Ecological Potential of Lake Types in Bulgaria S. Cheshmedjiev, D. Belkinova, R. Mladenov, I. Dimitrova-Dyulgerova & G. Gecheva To cite this article: S. Cheshmedjiev, D. Belkinova, R. Mladenov, I. Dimitrova-Dyulgerova & G. Gecheva (2010) Phytoplankton Based Assessment of the Ecological Status and Ecological Potential of Lake Types in Bulgaria, Biotechnology & Biotechnological Equipment, 24:sup1, 14-25, DOI: 10.1080/13102818.2010.10817803 To link to this article: https://doi.org/10.1080/13102818.2010.10817803 © 2010 Taylor and Francis Group, LLC Published online: 15 Apr 2014. Submit your article to this journal Article views: 107 Full Terms & Conditions of access and use can be found at https://www.tandfonline.com/action/journalInformation?journalCode=tbeq20 PHYTOPLANKTON BASED ASSESSMENT OF THE ECOLOGICAL STATUS AND ECOLOGICAL POTENTIAL OF LAKE TYPES IN BULGARIA S. Cheshmedjiev1, D. Belkinova2, R. Mladenov2, I. Dimitrova-Dyulgerova2, G. Gecheva2 1SI Eco Consult Ltd., Sofia, Bulgaria 2University of Plovdiv “Paisij Hilendarski”, Faculty of Biology, Plovdiv, Bulgaria Correspondence to: Ivanka Dimitrova-Dyulgerova E-mail: [email protected] ABSTRACT Research has been carried out of the main characteristics of phytoplankton communities in order to assess the ecological status and ecological potential of the types of lakes in Bulgaria, according to the requirements of WFD 2000/60/EC. Eighty lakes/reservoirs have been researched on the territory of the Republic of Bulgaria. The assessment was made on the basis of four main metrics (phytoplankton biovolume; Algae Groups Index; transparency, chlorophyll a) and three additional metrics (% Cyanobacteria; intensity of algal “bloom” and presence of toxic species). -

Species Composition and Toxic Potential of Cyanobacteria in Some Western Rhodopes Dams
ECOLOGIA BALKANICA 2018, Vol. 10, Issue 2 December 2018 pp. 111-121 Species Composition and Toxic Potential of Cyanobacteria in Some Western Rhodopes Dams Ivanka I. Teneva1*, Diyana Y. Basheva1, Tsvetelina R. Mladenova1, Plamen S. Stoyanov1, Detelina S. Belkinova2, Rumen D. Mladenov1 1 – University of Plovdiv “Paisii Hilendarski”, Faculty of Biology, Department of Botany and Teaching Methods in Biology, Plovdiv, BULGARIA 2 – Institute of Biodiversity and Ecosystem Research, Bulgarian Academy of Sciences, Sofia, BULGARIA *Corresponding author: [email protected] Abstract. Cyanobacteria are photosynthetic prokaryotes with cosmopolitan distribution. They are major producers of primary biomass and free oxygen in most of the freshwater and marine biomes on the planet. Under certain conditions of the environment, these organisms can evolve massively and cause the so-called "water blooms". Very often these blooms are toxic due to the ability of some cyanobacteria to produce dangerous toxins (cyanotoxins) with hepatotoxic, neurotoxic or dermatotoxic effects. This determines the cyanobacteria as an ecological risk for the aquatic ecosystems as well as a threat for the health of animals and humans. Climate changes also lead to an increase in the percentage of cyanobacterial blooms. Therefore, the investigation of the species composition of this group in the Bulgarian reservoirs, the tracking of the blooms frequencies as well as the assessment of their toxic potential is of great importance. Unfortunately, such data at this stage are scarce. This study presents data on the species composition and toxic potential of Cyanobacteria during the summer months of 2017 in five reservoirs (Batak, Dospat, Shiroka polyana, Golyam Beglik and Krichim dams) in the Western Rhodope Mountain. -
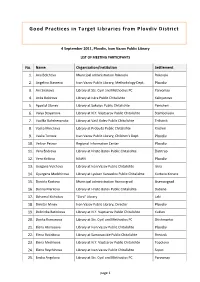
Good Practices in Target Libraries from Plovdiv District
Good Practices in Target Libraries from Plovdiv District 4 September 2011, Plovdiv, Ivan Vazov Public Library LIST OF MEETING PARTICIPANTS No. Name Organization/Institution Settlement 1. Ana Belcheva Municipal administration Rakovski Rakovski 2. Angelina Stavreva Ivan Vazov Public Library, Methodology Dept. Plovdiv 3. Ani Sirakova Library at Sts. Cyril and Methodius PC Parvomay 4. Anka Bekirova Library at Iskra Public Chitalishte Kaloyanovo 5. Apostol Stanev Library at Sokolov Public Chitalishte Panicheri 6. Valya Stoyanova Library at N.Y. Vaptsarov Public Chitalishte Stamboliyski 7. Vasilka Bahchevanska Library at Vasil Kolev Public Chitalishte Trilistnik 8. Vaska Mincheva Library at Probuda Public Chitalishte Krichim 9. Vaska Tonova Ivan Vazov Public Library, Children’s Dept. Plovdiv 10. Velizar Petrov Regional Information Center Plovdiv 11. Vera Endreva Library at Hristo Botev Public Chitalishte Zlatitrap 12. Vera Kirilova NAWV Plovdiv 13. Gergana Vulcheva Library at Ivan Vazov Public Chitalishte Iskra 14. Gyurgena Madzhirova Library at Lyuben Karavelov Public Chitalishte Kurtovo Konare 15. Daniela Kostova Municipal administration Asenovgrad Asenovgrad 16. Darina Markova Library at Hristo Botev Public Chitalishte Dabene 17. Dzhamal Kichukov “Zora” Library Laki 18. Dimitar Minev Ivan Vazov Public Library, Director Plovdiv 19. Dobrinka Batinkova Library at N.Y. Vaptsarov Public Chitalishte Kuklen 20. Donka Kumanova Library at Sts. Cyril and Methodius PC Shishmantsi 21. Elena Atanasova Library at Ivan Vazov Public Chitalishte Plovdiv 22. E lena Batinkova Library at Samorazvitie Public Chitalishte Brestnik 23. Elena Mechkova Library at N.Y. Vaptsarov Public Chitalishte Topolovo 24. Elena Raychinova Library at Ivan Vazov Public Chitalishte Sopot 25. Emilia Angelova Library at Sts. Cyril and Methodius PC Parvomay page 1 No. -

Xerotyphlops Vermicularis (MERREM, 1820), in the West Bulgarian Rhodope Mountains: Rediscovery After More Than 100 Years
200 SHORT NOTE HERPETOZOA 27 (3/4) Wien, 30. Jänner 2015 SHORT NOTE Xerotyphlops vermicularis (MERREM, 1820), in the west Bulgarian Rhodope Mountains: rediscovery after more than 100 years The Eurasian Blind Snake, Xerotyph - lops vermicularis (MERREM, 1820), the only representative of the snake family Typhlo - pidae (Scolecophidia) in Europe (gRil- liTSCH & gRilliTSCH 1993), is found in the southern parts of the Balkan Peninsula, specifically in former yugoslavia (Croatia, FyR Macedonia, Montenegro, Serbia), Albania, Bul garia, greece and Turkey (gRilliTSCH & gRilliTSCH 1993; gASC et al. 1997; gRilliTSCH et al. 1999) where it represents a chorotype element of the Turano-Mediterranean fauna (JABlONSki et al. 2012). However, the recent molecular data show that this traditionally accepted species is probably a species complex, since some populations from the Middle East show deep genetic divergences indicating their separate evolution since the end of Middle and late Miocene (kORNiliOS et al. 2012). This species prefers xerothermic habitats with deep, dry and soft (sandy) soil where it can burrow, typically rocky slopes with low, sparse bush vegetation, open areas with stones as well as cultivated fields (gRil liTSCH & gRilliTSCH 1993). The known edge of its distribution in the east of the Balkans is formed by several localities on Bulgarian territory (see BESH- kOv & NANEv 2006; STOJANOv et al. 2011), from where it was originally published only at the beginning of the 20th century (kOvA- CHEv 1912; CHiCHkOFF 1914). in Bul garia, this species has a scattered distribution at altitudes below 500 m above sea level. it is found only in the southern parts of the coun- try where it persisted from an earlier more extended distribution. -

Annex REPORT for 2019 UNDER the “HEALTH CARE” PRIORITY of the NATIONAL ROMA INTEGRATION STRATEGY of the REPUBLIC of BULGAR
Annex REPORT FOR 2019 UNDER THE “HEALTH CARE” PRIORITY of the NATIONAL ROMA INTEGRATION STRATEGY OF THE REPUBLIC OF BULGARIA 2012 - 2020 Operational objective: A national monitoring progress report has been prepared for implementation of Measure 1.1.2. “Performing obstetric and gynaecological examinations with mobile offices in settlements with compact Roma population”. During the period 01.07—20.11.2019, a total of 2,261 prophylactic medical examinations were carried out with the four mobile gynaecological offices to uninsured persons of Roma origin and to persons with difficult access to medical facilities, as 951 women were diagnosed with diseases. The implementation of the activity for each Regional Health Inspectorate is in accordance with an order of the Minister of Health to carry out not less than 500 examinations with each mobile gynaecological office. Financial resources of BGN 12,500 were allocated for each mobile unit, totalling BGN 50,000 for the four units. During the reporting period, the mobile gynecological offices were divided into four areas: Varna (the city of Varna, the village of Kamenar, the town of Ignatievo, the village of Staro Oryahovo, the village of Sindel, the village of Dubravino, the town of Provadia, the town of Devnya, the town of Suvorovo, the village of Chernevo, the town of Valchi Dol); Silistra (Tutrakan Municipality– the town of Tutrakan, the village of Tsar Samuel, the village of Nova Cherna, the village of Staro Selo, the village of Belitsa, the village of Preslavtsi, the village of Tarnovtsi, -

1 I. ANNEXES 1 Annex 6. Map and List of Rural Municipalities in Bulgaria
I. ANNEXES 1 Annex 6. Map and list of rural municipalities in Bulgaria (according to statistical definition). 1 List of rural municipalities in Bulgaria District District District District District District /Municipality /Municipality /Municipality /Municipality /Municipality /Municipality Blagoevgrad Vidin Lovech Plovdiv Smolyan Targovishte Bansko Belogradchik Apriltsi Brezovo Banite Antonovo Belitsa Boynitsa Letnitsa Kaloyanovo Borino Omurtag Gotse Delchev Bregovo Lukovit Karlovo Devin Opaka Garmen Gramada Teteven Krichim Dospat Popovo Kresna Dimovo Troyan Kuklen Zlatograd Haskovo Petrich Kula Ugarchin Laki Madan Ivaylovgrad Razlog Makresh Yablanitsa Maritsa Nedelino Lyubimets Sandanski Novo Selo Montana Perushtitsa Rudozem Madzharovo Satovcha Ruzhintsi Berkovitsa Parvomay Chepelare Mineralni bani Simitli Chuprene Boychinovtsi Rakovski Sofia - district Svilengrad Strumyani Vratsa Brusartsi Rodopi Anton Simeonovgrad Hadzhidimovo Borovan Varshets Sadovo Bozhurishte Stambolovo Yakoruda Byala Slatina Valchedram Sopot Botevgrad Topolovgrad Burgas Knezha Georgi Damyanovo Stamboliyski Godech Harmanli Aitos Kozloduy Lom Saedinenie Gorna Malina Shumen Kameno Krivodol Medkovets Hisarya Dolna banya Veliki Preslav Karnobat Mezdra Chiprovtsi Razgrad Dragoman Venets Malko Tarnovo Mizia Yakimovo Zavet Elin Pelin Varbitsa Nesebar Oryahovo Pazardzhik Isperih Etropole Kaolinovo Pomorie Roman Batak Kubrat Zlatitsa Kaspichan Primorsko Hayredin Belovo Loznitsa Ihtiman Nikola Kozlevo Ruen Gabrovo Bratsigovo Samuil Koprivshtitsa Novi Pazar Sozopol Dryanovo -

National Report Bulgaria
NATIONAL REPORT BULGARIA AGREEMENT ON THE CONSERVATION OF AFRICAN-EURASIAN MIGRATORY WATERBIRDS (The Hague, 1995) Implementation during the period 2006 and 2008 Contracting Party: BULGARIA Designated AEWA Administrative Authority: Ministry of Environment and Water Full name of the institution: Name and title of the head of the institution: Dzhevdet Chakarov - Minister Mailing address: 22, Maria Luisa Blvd Telephone: (+ 359 2) 988 25 77 Fax: (+ 359 2) 986 25 33 Email: [email protected] Name and title (if different) of the designated contact officer for AEWA matters: Valeri Georgiev Mailing address (if different) for the designated contact officer: 22, Maria Luisa Blvd Telephone: (+ 359 2) 940 6151 Fax: (+ 359 2) 940 6127 Email: [email protected] 2 Table of Contents 1. Overview of Action Plan implementation 5 2. Species conservation 6 Legal measures 6 Single Species Action Plans 8 Emergency measures 8 Re-establishments 8 Introductions 8 3. Habitat conservation 8 Habitat inventories 8 Conservation of areas 9 Rehabilitation and restoration 10 4. Management of human activities 11 Hunting 13 Eco-tourism 14 Other human activities 14 5. Research and monitoring 15 Status of research and monitoring programmes for species 15 6. Education and information 16 Training and development programmes 16 Raising public awareness 16 7. Final comments 17 8. Progress to implement Resolutions and Recommendations of the Meeting of the Parties 17 9. OPTIONAL SECTION – Planned and future actions 17 List of abbreviations and acronyms used in the -

Priority Public Investments for Wastewater Treatment and Landfill of Waste
Environmentally and Socially Sustainable Develonment Europe and Central Asia Region 32051 BULGARIA Public Disclosure Authorized ENVIRONMENTAL SEQUENCING STRATEGIES FOR EU ACCESSION PriorityPublic Investments for Wastewater Treatment and Landfill of Waste *t~~~~~~~~~~~~~~~~~~~~~~~ Public Disclosure Authorized IC- - ; s - o Fk - L - -. Public Disclosure Authorized The World Bank Public Disclosure Authorized May 2004 - "Wo BULGARIA ENVIRONMENTAL SEQUENCING STRATEGIES FOR EU ACCESSION Priority Public Investments for Wastewater Treatment and Landfill of Waste May 2004 Environmentally and Socially Sustainable Development Europe and Central Asia Region Report No. 27770 - BUL Thefindings, interpretationsand conclusions expressed here are those of the author(s) and do not necessarily reflect the views of the Board of Executive Directors of the World Bank or the governments they represent. Coverphoto is kindly provided by the external communication office of the World Bank County Office in Bulgaria. The report is printed on 30% post consumer recycledpaper. TABLE OF CONTENTS Acknowledgements ..................................................................... i Abbreviations and Acronyms ..................................................................... ii Summary ..................................................................... iiM Introduction.iii Wastewater.iv InstitutionalIssues .xvi Recommendations........... xvii Introduction ...................................................................... 1 Part I: The Strategic Settings for -

Management of Marine Protected Areas Management of Marine Protected Areas
Management of Marine Protected Areas Management of Marine Protected Areas A Network Perspective Edited by Paul D. Goriup This edition first published 2017 © 2017 John Wiley & Sons Ltd All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, except as permitted by law. Advice on how to obtain permission to reuse material from this title is available at www.wiley.com/go/permissions. The right of Paul D. Goriup to be identified as the author of the editorial material in this work has been asserted in accordance with law. Registered Office John Wiley & Sons Ltd, The Atrium, Southern Gate, Chichester, West Sussex, PO19 8SQ, UK Editorial Offices 111 River Street, Hoboken, NJ 07030, USA 9600 Garsington Road, Oxford, OX4 2DQ, UK The Atrium, Southern Gate, Chichester, West Sussex, PO19 8SQ, UK Boschstr. 12, 69469 Weinheim, Germany For details of our global editorial offices, customer services, and more information about Wiley products visit us at www.wiley.com. Wiley also publishes its books in a variety of electronic formats and by print‐on‐demand. Some content that appears in standard print versions of this book may not be available in other formats. Limit of Liability/Disclaimer of Warranty The publisher and the authors make no representations or warranties with respect to the accuracy or completeness of the contents of this work and specifically disclaim all warranties, including without limitation any implied warranties of fitness for a particular purpose. This work is sold with the understanding that the publisher is not engaged in rendering professional services. -

Project Mar Projet
IUCN Publications new scries N°5 Project Mar The conservation and management of temperate marshes, bogs and other wetlands SECOND VOLUME Projet Mar Conservation et aménagement des marécages, tourbières et autres milieux humides en zone tempérée DEUXIÈME VOLUME List of European and North African Wetlands of International Importance Liste des zones humides d'importance internationale en Europe et dans le Maghreb Project Mar The conservation and management of temperate marshes, bogs and other wetlands Projet Mar Conservation et aménagement des marécages, tourbières et autres milieux humides en zone tempérée Compiled by P. J. S. OLNEY and the staff of the IWRB/MAR Bureau Sponsored by IUCN, ICBP and IWRB Compilé par P. J. S. OLNEY et le personnel du Bureau BIRS/MAR Sous les auspices de l'UICN, du CIPO et du BIRS IUCN Publications new scries N°5 International Union for the Conservation of Nature and Natural Resources International Council for Bird Preservation International Wildfowl Research Bureau PROJECT MAR for the conservation and management of temperate wetlands List of European and North African wetlands of international importance Union Internationale pour la Conservation de la Nature et de ses Ressources Conseil International pour la Préservation des Oiseaux Bureau International de Recherches sur la Sauvagine PROJET MAR pour la conservation et l'aménagement des zones humides tempérées Liste des zones humides d'importance internationale en Europe et dans le Maghreb CONTENTS TABLE DES MATIERES Introduction (English) 7 Introduction (français) 13 Albania 21 Algérie 21 Austria 22 Belgique 24 Bulgaria 24 Czechoslovakia 25 Denmark 27 Espagne 30 Finland 33 France 34 Germany : Eastern part 41 Western part 43 Great Britain . -

Directory of Azov-Black Sea Coastal Wetlands
Directory of Azov-Black Sea Coastal Wetlands Kyiv–2003 Directory of Azov-Black Sea Coastal Wetlands: Revised and updated. — Kyiv: Wetlands International, 2003. — 235 pp., 81 maps. — ISBN 90 5882 9618 Published by the Black Sea Program of Wetlands International PO Box 82, Kiev-32, 01032, Ukraine E-mail: [email protected] Editor: Gennadiy Marushevsky Editing of English text: Rosie Ounsted Lay-out: Victor Melnychuk Photos on cover: Valeriy Siokhin, Vasiliy Kostyushin The presentation of material in this report and the geographical designations employed do not imply the expres- sion of any opinion whatsoever on the part of Wetlands International concerning the legal status of any coun- try, area or territory, or concerning the delimitation of its boundaries or frontiers. The publication is supported by Wetlands International through a grant from the Ministry of Agriculture, Nature Management and Fisheries of the Netherlands and the Ministry of Foreign Affairs of the Netherlands (MATRA Fund/Programme International Nature Management) ISBN 90 5882 9618 Copyright © 2003 Wetlands International, Kyiv, Ukraine All rights reserved CONTENTS CONTENTS3 6 7 13 14 15 16 22 22 24 26 28 30 32 35 37 40 43 45 46 54 54 56 58 58 59 61 62 64 64 66 67 68 70 71 76 80 80 82 84 85 86 86 86 89 90 90 91 91 93 Contents 3 94 99 99 100 101 103 104 106 107 109 111 113 114 119 119 126 130 132 135 139 142 148 149 152 153 155 157 157 158 160 162 164 164 165 170 170 172 173 175 177 179 180 182 184 186 188 191 193 196 198 199 201 202 4 Directory of Azov-Black Sea Coastal Wetlands 203 204 207 208 209 210 212 214 214 216 218 219 220 221 222 223 224 225 226 227 230 232 233 Contents 5 EDITORIAL AND ACKNOWLEDGEMENTS This Directory is based on the national reports prepared for the Wetlands International project ‘The Importance of Black Sea Coastal Wetlands in Particular for Migratory Waterbirds’, sponsored by the Netherlands Ministry of Agriculture, Nature Management and Fisheries. -

Groundwater Quality and Quantity in Europe
Technical report No 22 Groundwater quality and quantity in Europe Data and basic information Prepared by: A. Scheidleder, J. Grath, G. Winkler, U. Stärk, C. Koreimann and C. Gmeiner, Austrian Working Group on Water; P. Gravesen, Geological Survey of Denmark and Greenland; J. Leonard, International Office for Water; M. Elvira, Centro de Estudios y Experimentación de Obras Públicas; S. Nixon and J. Casillas, Water Research Centre; T. J. Lack, ETC-IW Leader July 1999 Project manager: Niels Thyssen European Environment Agency Cover design: Rolf Kuchling, EEA Legal notice The contents of this report do not necessarily reflect the official opinion of the European Communities or other European Communities institutions. Neither the European Environment Agency nor any person or company acting on the behalf of the Agency is responsible for the use that may be made of the information contained in this report. A great deal of additional information on the European Union is available on the Internet. It can be accessed through the Europa server (http://europa.eu.int) ©EEA, Copenhagen, 1999 Reproduction is authorised provided the source is acknowledged Printed in Copenhagen Printed on recycled and chlorine-free bleached paper European Environment Agency Kongens Nytorv 6 DK-1050 Copenhagen K Denmark Tel: +45 33 36 71 00 Fax: +45 33 36 71 99 E-mail: [email protected] 2 Table of contents 1. Introduction ....................................................................................................4 2. Groundwater quality and quantity in Europe ..................................................5