United States Patent Office
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Gsaüiiveiwibte
'C or GSAÜIIVEIWIBTE CUflIIITIIIi MïtiTHI AJIAlfïïf ,7 -' y/ . •'• .'7. 's -i, . \ STELLINGEN BEHORENDE BIJ HEJ FROEFSCHRIffT VAN R. FURLER 1. De episoomtheorie over het ontstaan van het mitochon- drion is weinig plausibel. R.A, Ratt and H.R. Mahler, Science 221 O972),575 2, Doordat S. Cirendini et al. de dragergassnelheid aan 'aet einde van een chromatografische kolom gebruiken, ontstaat een geflatteerd beeld van de weergegeven re- sultaten. Tevens is het niet mogelijk een dragergas- snelheid te berekenen zonder dat men de interstitiële porositeit kent» S, Cirendini, J. Vermont, J.C. Gressin and CL. Guilleain , J. Chromat. 84 (1973),24 3. De in de mode zijnde bepaling van RNA-moleculair ge- wichten door metingen aan formaldehyde behandelde RNA's berust op dubieuze aannamen. J.M. Kaper and M.E. v/aterworth,Virology 51 (1973),183 T.O. Diener and D.R. Smith, Virology *£ (^973), 359 M.M. El Manna and G. Bruening, Virology 56 (1973),198 4, Op grond van de zeer grote verschillen in stralingska- rakteristiek van de isotopen 1-131 en 1-123 is het streven van isotopenproducenten om een zo 'schoon' mogelijk 1-123 voor diagnostische doeleinden te leve- ren in strijd met de volksgezondheid, doordat de ver- tragingen#die dit oplevert onnodige stralingsbelasting voor patiënten veroorzaakt. H. ïlishiyama et al. J.Nucl.Med. 1£ (1974),261 5« De analogie die Gilbert et al. opmerken tussen de "exchange peak" in de kolom vloaistofchromatografie met behulp van ionenwisselaar en de luchtpi.ek bij gaschromatografie is twijfelachtig. T.W. Gilbert and R.A, Dobbs, Analyt.Chem. 45 (1>73), 1390. -

Circular of the Bureau of Standards No. 539 Volume 10: Standard X-Ray
:ationa.u d H.W. BIS <T be Libra.ry, Reisrence book not to 1965 JVPR 1 6 from ibe lib s ary. taken NBS C | RCULAR 539 VOLUME 10 Standard X-ray Diffraction Powder Patterns UNITED STATES DEPARTMENT OF COMMERCE NATIONAL BUREAU OF STANDARDS THE NATIONAL BUREAU OF STANDARDS Functions and Activities The Functions of the National Bureau of Standards are set forth in the Act of Congress, March 3, 1901, as amended by Congress in Public Law 619, 1950. These include the development and maintenance of the national standards of measurement and the provision of means and methods for making measurements consistent with these standards; the determination of physical constants and properties of materials; the development of methods and instruments for testing materials, devices, and structures; advisory services to government agencies on scientific and technical problems; in- vention and development of devices to serve special needs of the Government; and the development of standard practices, codes, and specifications. The work includes basic and applied research, development, engineering, instrumentation, testing, evaluation, calibration services, and various consultation and information services. Research projects are also performed for other government agencies when the work relates to and supplements the basic program of the Bureau or when the Bureau’s unique competence is required. The scope of activities is suggested by the listing of divisions and sections on the inside of the back cover. Publications The results of the Bureau’s work take the form of either actual equipment and devices or pub- lished papers. These papers appear either in the Bureau’s own series of publications or in the journals of professional and scientific societies. -

4. Inorganic Flame Retardants. Plastics Can Be Given Flame Retardant Characteristics by Introducing Elements of Organic, Inorganic and Halogen Origin
4. Inorganic Flame Retardants. Plastics can be given flame retardant characteristics by introducing elements of organic, inorganic and halogen origin. Such elements include magnesium, aluminium, phosphorous, molybdenum, antimony, tin, chlorine and bromine. Flame retardants are added in either the manufacturing step of the polymer or the compounding step of the polymeric article. Phosphorous bromine and chlorine are usually included as some organic compound. Inorganic flame retardants are usually added together with other flame retardants to provide a more efficient flame retardant action through synergism. Halogen flame retardants usually need an addition of about 40% in order to be effective, and this affects the properties of the polymer quite negatively. Structural integrity of the polymer article is often very important, and a drastic decrease in strength and other mechanical properties is simply not acceptable. The efficiency of halogen flame retardants is often enhanced by the addition of inorganic flame retardants. A smaller mass percentage halogen flame retardant is now needed, so the adverse effect on the polymer properties is also reduced (Touval, 1993) . 4.1 Antimony Compounds The antimony compounds used for flame retardancy include antimony trioxide, antimony pentoxide and antimony-metal compounds. In 1990 in the United States alone, the use of antimony trioxide amounted to 20 000 metric tons just for the flame retardancy of plastics. Antimony oxide is readily found in nature but in very impure form. This is not suitable for 29 direct use as flame retardant, so antimony oxide is often rather produced from antimony metal. There are therefore many different grades of antimony oxide that can be used for flame retardants. -

WO 2015/121485 Al 20 August 2015 (20.08.2015) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2015/121485 Al 20 August 2015 (20.08.2015) P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every B01J 23/18 (2006.01) B01J 35/02 (2006.01) kind of national protection available): AE, AG, AL, AM, B01J 23/22 (2006.01) C07C 15/24 (2006.01) AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, B01J 27/198 (2006.01) B01J 37/00 (2006.01) BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, B01J 35/00 (2006.01) DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, (21) International Application Number: KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, PCT/EP2015/053270 MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, (22) International Filing Date: PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, 17 February 2015 (17.02.2015) SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (25) Filing Language: English (84) Designated States (unless otherwise indicated, for every (26) Publication Language: English kind of regional protection available): ARIPO (BW, GH, (30) Priority Data: GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, 14155332. -

Properties and Human Exposure; Sanford Garner; Roc; Jan. 24, 2018
Draft RoC Monograph on Antimony Trioxide Properties and Human Exposure Sanford Garner, PhD Integrated Laboratory Systems, Inc. Contractor supporting the Office of the Report on Carcinogens National Institute of Environmental Health Sciences January 24, 2018 Properties Antimony and antimony compounds • Antimony is a metalloid found in nature in over 100 mineral species – Exists as four oxidation states: -3, 0, +3 and +5 • +3 (trivalent) and +5 (pentavalent) are most common in environmental, biological, and geochemical systems – Antimony species can undergo transformation during manufacturing processes, in the environment, or in vivo • Elemental antimony is a silver-white metal used to make alloys • Antimony(III) trioxide exists as an odorless white powder or polymorphic crystals Properties Solubility of antimony oxides and antimony metal is higher in biological fluids than in water • Antimony trioxide: 3.3 mg/L in water • Antimony pentoxide: 0.043 mg/L in water • Antimony metal: Insoluble in water Source: ECHA Registration Dossiers for diantimony pentoxide and diantimony trioxide. Properties and Human Exposure Human Exposure Human Exposure A significant number of people in the United States are exposed to antimony(III) trioxide based on: • Consumption (~ 70 million lb/yr; 1 producer and 10 importers reported in the United States) in manufacturing • Widespread use in industrial applications (e.g., 273 companies in the flame retardant industry) • Occupational exposure • General population exposure – Consumer products – Environmental exposure Uses of Antimony(III) Trioxide Antimony(III) trioxide is the most commercially significant form of processed antimony • Workers in formulation, processing, and manufacturing of consumer products are exposed to antimony(III) trioxide Consumer Formulation Processing products flame retardant e.g., furniture, flame retardant plastics (including electrical and synergist PVC), textiles, electronic equipment rubbers e.g., PET containers PET packaging and PET catalyst for water, soft drinks, fibers etc. -

CLARC Excerpt
Washington State Department of Ecology - CLARC Air Table (Methods B and C) - February 2021 February 2021 S S CPFi S CPFo S Air Air RfC o RfDi o Inhalation RfDo o Oral o Air Air Method C Method C Inhalation u Inhalation IUR u Cancer Oral u Cancer u Method B Method B Noncancer Cancer Reference Reference Inhalation Potency Reference Potency Noncancer Cancer (Eq. 750-1 (Eq. 750-2 Chemical Data Links to r r r r Concentration c Dose Unit Risk c Factor Dose c Factor c (Eq. 750-1) (Eq. 750-2) adjusted) adjusted) 3 3 -1 CAS No. Group Chemical Name Important Notes (mg/m ) e (mg/kg-day) (µg/m ) e (kg-day/mg) (mg/kg-day) e (kg-day/mg) e (µg/m³) (µg/m³) (µg/m³) (µg/m³) 83-32-9 PAHs acenaphthene 6.00E-02 I 30560-19-1 Pesticides acephate 1.20E-03 O 75-07-0 VOCs acetaldehyde 9.00E-03 I 2.57E-03 2.20E-06 I 7.70E-03 4.10E+00 1.10E+00 9.00E+00 1.10E+01 34256-82-1 Pesticides acetochlor 2.00E-02 I 67-64-1 VOCs acetone 3.10E+01 A 8.86E+00 9.00E-01 I 1.40E+04 3.10E+04 75-86-5 VOCs acetone cyanohydrin 2.00E-03 X 5.71E-04 9.10E-01 2.00E+00 75-05-8 VOCs acetonitrile 6.00E-02 I 1.71E-02 2.70E+01 6.00E+01 98-86-2 SVOCs acetophenone 1.00E-01 I 62476-59-9 Herbicides acifluorfen, sodium 1.30E-02 I 107-02-8 VOCs acrolein 2.00E-05 I 5.71E-06 5.00E-04 I 9.10E-03 2.00E-02 79-06-1 VOCs acrylamide 6.00E-03 I 1.71E-03 1.00E-04 I-M 3.50E-01 2.00E-03 I 5.00E-01 I-M 2.70E+00 6.60E-03 6.00E+00 2.50E-01 79-10-7 VOCs acrylic acid 1.00E-03 I 2.86E-04 5.00E-01 I 4.60E-01 1.00E+00 107-13-1 VOCs acrylonitrile 2.00E-03 I 5.71E-04 6.80E-05 I 2.38E-01 4.00E-02 A 5.40E-01 I 9.10E-01 3.70E-02 -

123. Antimony
1998:11 The Nordic Expert Group for Criteria Documentation of Health Risks from Chemicals 123. Antimony John Erik Berg Knut Skyberg Nordic Council of Ministers arbete och hälsa vetenskaplig skriftserie ISBN 91–7045–471–x ISSN 0346–7821 http://www.niwl.se/ah/ah.htm National Institute for Working Life National Institute for Working Life The National Institute for Working Life is Sweden's center for research and development on labour market, working life and work environment. Diffusion of infor- mation, training and teaching, local development and international collaboration are other important issues for the Institute. The R&D competence will be found in the following areas: Labour market and labour legislation, work organization and production technology, psychosocial working conditions, occupational medicine, allergy, effects on the nervous system, ergonomics, work environment technology and musculoskeletal disorders, chemical hazards and toxicology. A total of about 470 people work at the Institute, around 370 with research and development. The Institute’s staff includes 32 professors and in total 122 persons with a postdoctoral degree. The National Institute for Working Life has a large international collaboration in R&D, including a number of projects within the EC Framework Programme for Research and Technology Development. ARBETE OCH HÄLSA Redaktör: Anders Kjellberg Redaktionskommitté: Anders Colmsjö och Ewa Wigaeus Hjelm © Arbetslivsinstitutet & författarna 1998 Arbetslivsinstitutet, 171 84 Solna, Sverige ISBN 91–7045–471–X ISSN 0346-7821 Tryckt hos CM Gruppen Preface The Nordic Council is an intergovernmental collaborative body for the five countries, Denmark, Finland, Iceland, Norway and Sweden. One of the committees, the Nordic Senior Executive Committee for Occupational Environmental Matters, initiated a project in order to produce criteria documents to be used by the regulatory authorities in the Nordic countries as a scientific basis for the setting of national occupational exposure limits. -
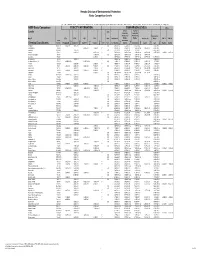
Basic Comparison Levels
Nevada Division of Environmental Protection Basic Comparison Levels Key: I=IRIS; P= PPRTV; N=NCEA; H=HEAST; A=ATSDR; O=Other Documents; CA=CalEPA S=Surrogate X=Appendix PPRTV E=Based on TEF scheme r=Route Extra Key: C = Cancer endpoint; N = Noncancer endpoint; sat = Saturation Limit; max = Ceiling Limit NDEP Basic Comparison TOXICITY INFORMATION COMPARISON LEVELS LBCLs Indoor Outdoor Levels Skin Industrial/ Industrial/ Commercial Commercial Residential May-17 SFo RfDo IUR RfCi Abs. Residential Worker Worker Ambient Air Water DAF 1 DAF 20 CAS w/o Dermal Chemical Constituents Number 1/(mg/kg-d) (mg/kg-d) (ug/m3)-1 (mg/m3) VOCc Soils Soil (mg/kg) (mg/kg) Soil (mg/kg) (µg/m3) (µg/l) (mg/kg) (mg/kg) Key Key key Key Key Key Key Key Key Acephate 30560-19-1 8.70E-03 I 4.00E-03 I 0.10 5.59E+01 C 7.52E+02 C 2.95E+02 C 7.73E+00 C Acetaldehyde 75-07-0 2.20E-06 I 9.00E-03 I V 1.23E+01 C 5.35E+01 C 1.00E+05 max 1.28E+00 C 2.55E+00 C Acetochlor 34256-82-1 2.00E-02 I 0.10 1.23E+03 N 4.67E+04 N 1.83E+04 N 6.67E+02 N Acetone 67-64-1 9.00E-01 I 3.10E+01 A V 7.04E+04 N 1.00E+05 max 1.00E+05 max 3.23E+04 N 2.05E+04 N 8.00E-01 1.60E+01 Acetone Cyanohydrin 75-86-5 2.00E-03 X 0.10 1.00E+05 max 1.00E+05 max 1.00E+05 max 2.09E+00 N Acetonitrile 75-05-8 6.00E-02 I V 1.00E+05 max 3.75E+03 N 1.00E+05 max 6.26E+01 N 1.25E+02 N Acetophenone 98-86-2 1.00E-01 I V 2.52E+03 sat 2.52E+03 sat 2.52E+03 sat 3.34E+03 N Acetylaminofluorene, 2- 53-96-3 3.80E+00 CA 1.30E-03 CA 0.10 1.28E-01 C 1.72E+00 C 6.75E-01 C 2.16E-03 C 1.77E-02 C Acrolein 107-02-8 5.00E-04 I 2.00E-05 I V -

Toxicity Summary for Antimony December 1992
TOXICITY SUMMARY FOR ANTIMONY DECEMBER 1992 Prepared by Robert A. Young, Ph.D., D.A.B.T. Chemical Hazard Evaluation and Communication Group Biomedical and Environmental Information Analysis Section Health and Safety Research Division Oak Ridge National Laboratory* Oak Ridge, Tennessee Prepared for OAK RIDGE RESERVATION ENVIRONMENTAL RESTORATION PROGRAM *Managed by Martin Marietta Energy Systems, Inc., for the U.S. Department of Energy under Contract No. DE-AC05-84OR21400 This page intentionally left blank. EXECUTIVE SUMMARY Antimony (Sb) is a naturally occurring metal that is used in various manufacturing processes. It exists in valence states of 3 and 5 (Budavari, 1989; ATSDR, 1990). Antimony is a common urban air pollutant (Beliles, 1979). Exposure to antimony may be via inhalation, oral and dermal routes (ATSDR, 1990). Antimony is sparingly absorbed following ingestion or inhalation (Felicetti et al., 1974a; Gerber et al., 1982; ATSDR, 1990). Both gastrointestinal and pulmonary absorption are a function of compound solubility. Antimony is transported in the blood, its distribution varying among species and dependent on its valence state (Felicetti et al., 1974b). Antimony is not metabolized but may bind to macromolecules and react covalently with sulfhydryl and phosphate groups (ATSDR, 1990). Excretion of antimony is primarily via the urine and feces, and is also dependent upon valence state (Cooper et al., 1968; Ludersdorf et al., 1987; ATSDR, 1990). Acute oral exposure of humans and animals to high doses of antimony or antimony-containing compounds (antimonials) may cause gastrointestinal disorders (vomiting, diarrhea), respiratory difficulties, and death at extremely high doses (Bradley and Frederick, 1941; Beliles, 1979; ATSDR, 1990). -
![Antimony Trioxide [CAS No. 1309-64-4]](https://docslib.b-cdn.net/cover/1805/antimony-trioxide-cas-no-1309-64-4-1681805.webp)
Antimony Trioxide [CAS No. 1309-64-4]
Antimony Trioxide [CAS No. 1309-64-4] Brief Review of Toxicological Literature Prepared for National Toxicology Program (NTP) National Institute of Environmental Health Sciences (NIEHS) National Institutes of Health U.S. Department of Health and Human Services Contract No. N01-ES-35515 Project Officer: Scott A. Masten, Ph.D. NTP/NIEHS Research Triangle Park, North Carolina Prepared by Integrated Laboratory Systems, Inc. Research Triangle Park, North Carolina July 2005 Chemical Name: Antimony Trioxide CAS RN: 1309-64-4 Formula: Sb2O3 Basis for Nomination: Antimony trioxide was nominated by the National Institute of Environmental Health Sciences for chronic toxicity, cardiotoxicity and carcinogenicity studies due to the potential for substantial human exposure in occupational settings and lack of adequate two-year exposure carcinogenicity studies. Additional studies of antimony trioxide are of interest, in lieu of studies with antimony trisulfide or another antimony compound, considering the higher volume of use and magnitude of human exposure, and the lack of two- year exposure carcinogenicity studies for any antimony compound by any route of administration. Antimony trisulfide was nominated to the NTP by the National Cancer Institute in 2002 for carcinogenicity studies (http://ntp.niehs.nih.gov/ntpweb/index.cfm?objectid=25BEBA08-BDB7-CEBA- FC56EAD78615ADCF). Subsequent to the nomination review process, the NTP concluded that antimony trisulfide should not be studied further at this time for the following reasons: 1) antimony trisulfide does not appear to represent a major form of antimony to which humans are exposed; 2) carcinogenicity studies of antimony trisulfide is unlikely to lead to a sufficient understanding of the carcinogenicity hazard for antimony compounds as a group; and 3) there is concern regarding the safe handling of pure antimony trisulfide in experimental settings due to its flammable/combustible nature. -

Positively Charged Antimony Pentoxide Sol and Preparation Thereof
~" ' Nil II II II III Ml Nil INI Ml Ml J European Patent Office *m _ _ _ © Publication number: 0 197 503 B1 Office_„. europeen des brevets EUROPEAN PATENT SPECIFICATION © Date of publication of patent specification: 15.07.92 © Int. CI.5: C01G 30/00 © Application number: 86104529.2 @ Date of filing: 03.04.86 © Positively charged antimony pentoxlde sol and preparation thereof. ® Priority: 03.04.85 JP 70720/85 Qj) Proprietor: NISSAN CHEMICAL INDUSTRIES LTD. @ Date of publication of application: 3-7-1, Kanda Nlshlkl-cho 15.10.86 Bulletin 86/42 Chlyoda-ku Tokyo(JP) © Publication of the grant of the patent: @ Inventor: Watanabe, Yoshltane 15.07.92 Bulletin 92/29 Juneshlon Hlral No. 911 3-5-1, Hlral Edogawa-ku Tokyo(JP) © Designated Contracting States: Inventor: Teranlshl, Masayukl DE FR GB IT 7-27-13, Takanedal Funabashl-shl Chlba-ken(JP) © References cited: Inventor: Suzuki, Keltaro DE-A- 2 931 523 1-5-16-201, Narashlno US-A- 4 075 032 Funabashl-shl Chlba-ken(JP) US-A- 4 100 076 US-A- 4 100 077 US-A- 4 341 655 © Representative: Lehn, Werner, Dipl.-lng. et al Hoffmann, Eltle & Partner Patentanwalte Ar- abellastrasse 4 W-8000 Munchen 81 (DE) 00 CO o m O Note: Within nine months from the publication of the mention of the grant of the European patent, any person ^ may give notice to the European Patent Office of opposition to the European patent granted. Notice of opposition qj shall be filed in a written reasoned statement. It shall not be deemed to have been filed until the opposition fee has been paid (Art. -

Chemical Names and CAS Numbers Final
Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number C3H8O 1‐propanol C4H7BrO2 2‐bromobutyric acid 80‐58‐0 GeH3COOH 2‐germaacetic acid C4H10 2‐methylpropane 75‐28‐5 C3H8O 2‐propanol 67‐63‐0 C6H10O3 4‐acetylbutyric acid 448671 C4H7BrO2 4‐bromobutyric acid 2623‐87‐2 CH3CHO acetaldehyde CH3CONH2 acetamide C8H9NO2 acetaminophen 103‐90‐2 − C2H3O2 acetate ion − CH3COO acetate ion C2H4O2 acetic acid 64‐19‐7 CH3COOH acetic acid (CH3)2CO acetone CH3COCl acetyl chloride C2H2 acetylene 74‐86‐2 HCCH acetylene C9H8O4 acetylsalicylic acid 50‐78‐2 H2C(CH)CN acrylonitrile C3H7NO2 Ala C3H7NO2 alanine 56‐41‐7 NaAlSi3O3 albite AlSb aluminium antimonide 25152‐52‐7 AlAs aluminium arsenide 22831‐42‐1 AlBO2 aluminium borate 61279‐70‐7 AlBO aluminium boron oxide 12041‐48‐4 AlBr3 aluminium bromide 7727‐15‐3 AlBr3•6H2O aluminium bromide hexahydrate 2149397 AlCl4Cs aluminium caesium tetrachloride 17992‐03‐9 AlCl3 aluminium chloride (anhydrous) 7446‐70‐0 AlCl3•6H2O aluminium chloride hexahydrate 7784‐13‐6 AlClO aluminium chloride oxide 13596‐11‐7 AlB2 aluminium diboride 12041‐50‐8 AlF2 aluminium difluoride 13569‐23‐8 AlF2O aluminium difluoride oxide 38344‐66‐0 AlB12 aluminium dodecaboride 12041‐54‐2 Al2F6 aluminium fluoride 17949‐86‐9 AlF3 aluminium fluoride 7784‐18‐1 Al(CHO2)3 aluminium formate 7360‐53‐4 1 of 75 Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number Al(OH)3 aluminium hydroxide 21645‐51‐2 Al2I6 aluminium iodide 18898‐35‐6 AlI3 aluminium iodide 7784‐23‐8 AlBr aluminium monobromide 22359‐97‐3 AlCl aluminium monochloride