Time–Outcome Relationship in Acute Large-Vessel Occlusion Exists Across All Ages
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Osaka Train Route Map Lastupdate May.22.2021 Kanmaki Minase Takatsuki Tokaido(Kyoto) Line Y E
Shimamoto X Osaka Train Route map LastUpdate May.22.2021 Kanmaki Minase Takatsuki Tokaido(Kyoto) Line Y e n Saitonishi i Z Hankyu Minoo Line L o Settsutonda Takatsukishi t i Minoo a Toyokawa Tonda Kuzuha S A l i Hankyu-Takarazuka Line a JRSojiji r o n O Makiochi o Handaibyoinmae Gotenyama m Ikeda Sakurai a Sojiji Makino k a IshibashiHandaimae Kitasenri s Koenhigashiguchi Ibaraki Ibarakishi Hirakatashi O ShibaharaHandaimae Shoji Unobe Hirakatakoen Miyanosaka OsakaMonorail Line Hotarugaike Senrichuo Yamada BanpakuKinenKoen Minamiibaraki Hoshigaoka B Momoyamadai Minamisenri Senrioka Kozenji e n i L Osakakuko Toyonaka Sawaragi Muranno Nagao o n e a t Ryokochikoen n i a L K i Okamachi r Senriyama Kishibe Shojaku Settsushi Settsu Korien Kozu Fujisaka n n a e e h i n S i e e L n u K i y o L Sone Kandaimae e Katanoshi Tsuda k e k n n o i e u t a L y r o k H r y n a Osaka International a K k e Toyotsu h Kawachinomori u a n i i u u s Airport(ITM) e Hankyu Takarazuka Line y L o t K k l a i t n t a i a r K c Suita H o Hoshida Kawachiiwafune c n Tokaido(Kyoto) Line o e Hattoritenjin Aikawa M Minamisettsu Neyagawashi Kisaichi e a f k f Suita Itakano a s e O e Esaka MinamiSuita Neyagawakoen r r Zuiko4 P P N Shonai Kamishinjo Sonoda Higashiyodogawa Shimoshinjo Kayashima o o Shinobugaoka Dainichi t g Tokaido-Shinkansen R Imazatosuji Line o o Higashimikuni Daidotoyosato Moriguchi Owada y y Mikuni Awaji Taishibashiimaichi K Kashima Kanzakigawa Moriguchishi H Kadomashi P JRAwaji Nishisanso Furukawabashi ShinOsaka JR Osakahigashi Line Q Sozenji Senbayashiomiya -

Immunohistochemical Detection of WT1 Protein in a Variety of Cancer Cells
Modern Pathology (2006) 19, 804–814 & 2006 USCAP, Inc All rights reserved 0893-3952/06 $30.00 www.modernpathology.org Immunohistochemical detection of WT1 protein in a variety of cancer cells Shin-ichi Nakatsuka1, Yusuke Oji2, Tetsuya Horiuchi3, Takayoshi Kanda4, Michio Kitagawa5, Tamotsu Takeuchi6, Kiyoshi Kawano7, Yuko Kuwae8, Akira Yamauchi9, Meinoshin Okumura10, Yayoi Kitamura2, Yoshihiro Oka11, Ichiro Kawase11, Haruo Sugiyama12 and Katsuyuki Aozasa13 1Department of Clinical Laboratory, National Hospital Organization Osaka Minami Medical Center, Kawachinagano, Osaka, Japan; 2Department of Biomedical Informatics, Osaka University Graduate School of Medicine, Suita, Osaka, Japan; 3Department of Surgery, National Hospital Organization Osaka Minami Medical Center, Kawachinagano, Osaka, Japan; 4Department of Gynecology, National Hospital Organization Osaka Minami Medical Center, Kawachinagano, Osaka, Japan; 5Department of Urology, National Hospital Organization Osaka Minami Medical Center, Kawachinagano, Osaka, Japan; 6Department of Pathology, Kochi Medical School, Kohasu, Oko-cho, Nankoku City, Kochi, Japan; 7Department of Pathology, Osaka Rosai Hospital, Sakai, Osaka, Japan; 8Department of Pathology, Osaka Medical Center and Research Institute of Maternal and Child Health, Izumi, Osaka, Japan; 9Department of Cell Regulation, Faculty of Medicine, Kagawa University, Miki-cho, Kida-gun, Kagawa, Japan; 10Department of Surgery, Osaka University Graduate School of Medicine, Suita, Osaka, Japan; 11Department of Molecular Medicine, Osaka University Graduate School of Medicine, Suita, Osaka, Japan; 12Department of Functional Diagnostic Science, Osaka University Graduate School of Medicine, Suita, Osaka, Japan and 13Department of Pathology, Osaka University Graduate School of Medicine, Suita, Osaka, Japan WT1 was first identified as a tumor suppressor involved in the development of Wilms’ tumor. Recently, oncogenic properties of WT1 have been demonstrated in various hematological malignancies and solid tumors. -

Essentials for Living in Osaka (English)
~Guidebook for Foreign Residents~ Essentials for Living in Osaka (English) Osaka Foundation of International Exchange October 2018 Revised Edition Essentials for Living in Osaka Table of Contents Index by Category ⅠEmergency Measures ・・・1 1. Emergency Telephone Numbers 2. In Case of Emergency (Fire, Sudden Sickness and Crime) Fire; Sudden Illness & Injury etc.; Crime Victim, Phoning for Assistance; Body Parts 3. Precautions against Natural Disasters Typhoons, Earthquakes, Collecting Information on Natural Disasters; Evacuation Areas ⅡHealth and Medical Care ・・・8 1. Medical Care (Use of medical institutions) Medical Care in Japan; Medical Institutions; Hospital Admission; Hospitals with Foreign Language Speaking Staff; Injury or Sickness at Night or during Holidays 2. Medical Insurance (National Health Insurance, Nursing Care Insurance and others) Medical Insurance in Japan; National Health Insurance; Latter-Stage Elderly Healthcare Insurance System; Nursing Care Insurance (Kaigo Hoken) 3. Health Management Public Health Center (Hokenjo); Municipal Medical Health Center (Medical Care and Health) Ⅲ Daily Life and Housing ・・・16 1. Looking for Housing Applying for Prefectural Housing; Other Public Housing; Looking for Private Housing 2. Moving Out and Leaving Japan Procedures at Your Old Residence Before Moving; After Moving into a New Residence; When You Leave Japan 3. Water Service Application; Water Rates; Points of Concern in Winter 4. Electricity Electricity in Japan; Application for Electrical Service; Payment; Notice of the Amount of Electricity Used 5. Gas Types of Gas; Gas Leakage; Gas Usage Notice and Payment Receipt 6. Garbage Garbage Disposal; How to Dispose of Other Types of Garbage 7. Daily Life Manners for Living in Japan; Consumer Affairs 8. When You Face Problems in Life Ⅳ Residency Management System・Basic Resident Registration System for Foreign Nationals・Marriage・Divorce ・・・27 1. -

What Is Osaka University Doing in ASEAN Countries?
Osaka University ASEAN Center for Academic Initiatives Contact address: Room C, 10th Floor, Serm-Mit Tower, Sukhumvit 21 (Soi Asok), Klongtoey-nua, Wattana, Bangkok 10110, Thailand http://www.bangkok.overseas.osaka-u.ac.jp/ Osaka University was founded in 1931 as the 6th imperial university of Japan through strong demand from the business and government sectors of Osaka, as well as the people of Osaka City and Prefecture. Now Osaka University holds 11 schools,16 graduate schools and 25 research centers and institutes with 15,250 undergraduate and 8,054 graduate students including 2,480 international students and 3,541 full-time academic and 3,113 full- time non-academic staff. It also has 4 libraries and 2 university hospitals; 3 campuses in Suita, Toyonaka, and Minoh. What is Osaka University doing in ASEAN countries? ASEAN Center for Academic Initiatives is one of the four overseas centers of Osaka University. The mission is to bridge Osaka U with ASEAN universities and academics, and to encourage “study in Osaka University” to ASEAN students. International Center for Biotechnology operates the Collaborative Research Center for Bioscience and Biotechnology in Mahidol University to promote joint researches with Thai universities such as CU, MU, KU and KMUTT, and offers the international educational programs under the support of Japanese Government, UNESCO and Thai universities. Research Institute for Microbial Diseases operates Thailand-Japan Research Collaboration Center on Emerging and Re-emerging Infections (RCC-ERI) under the Japanese national project. RCC-ERI carries out the qualified researches on infectious bacteria and viruses specific in tropical region. It has also research laboratories in Mahidol University. -

Clinical Trial of Step-Up Exercise Therapy Using DVD for Patients with Hip Osteoarthritis
Abstracts / Osteoarthritis and Cartilage 20 (2012) S54–S296 S265 Symmetry Ratios (Operated / Non-Operated Limb) Symmetry Group (Pre-TKA) Symmetry Group (Post-Rehabilitation) Strengthening Group (Post-Rehabilitation) Knee Flexion Excursion Symmetry Ratio 0.5 +/- 0.3 1.1 +/- 1.0 0.7 +/- 0.4 Peak Knee Flexion Moment Symmetry Ratio 0.7 +/- 0.6 1.2 +/- 0.8 0.8 +/- 0.5 Peak Knee Flexion During Stance Symmetry Ratio 0.8 +/- 0.8 1.0 +/- 0.3 0.8 +/- 0.2 523 comments were also related with encouragement of correct under- CLINICAL TRIAL OF STEP-UP EXERCISE THERAPY USING DVD FOR standing of exercise using DVD. PATIENTS WITH HIP OSTEOARTHRITIS Y. Uesugi 1, J. Koyanagi 2, T. Nishii 2, S. Hayashi 3, T. Fujishiro 3, K. Takagi 4,R. Yamaguchi 5, T. Nishiyama 3. 1 Kobe Univ. Graduate Sch. of Hlth.Sci., Kobe, Japan; 2 Osaka Univ. Graduate Sch. of Med., Suita, Japan; 3 Kobe Univ. Sch. of Med., Kobe, Japan; 4 Dept. of Rehabilitation, Osaka Univ. Hosp., Suita, Japan; 5 Div. of Rehabilitation, Kobe Univ. Hosp., Kobe, Japan Purpose: Efficacy of exercise treatment for osteoarthritis (OA) has been reported controversially. We developed a new exercise program designed as several step-up stages in accordance with patient disease activity, using a digital video disc (DVD). In this study, we analyzed the efficacy of this step-up exercise therapy program using DVD for the hip OA patients. Methods: We prospectively recruited 45 patients (male1, female44) with symptomatic hip OA were included. All participants provided informed consent to the study which was approved by the Institutional Review Board. -

Storm Warning (Bofu-Keiho / 暴 風警報) Or an Emergency Warning (Tokubetsu-Keiho / 特別警報)
Class Cancellation due to Weather Warnings: Storm Warning (Bofu-keiho / 暴 風警報) or an Emergency Warning (Tokubetsu-keiho / 特別警報) At the moment, a typhoon is approaching Japan. Classes will be cancelled if any of the above warnings are issued. You can confirm the details of when class cancellation may occur according to areas and municipalities where warnings have been issued, and when the warning has been lifted on the following homepage or the table below. Kwansei Gakuin University Website Undergraduate: http://www.kwansei.ac.jp/a_affairs/a_affairs_000850.html Graduate : http://www.kwansei.ac.jp/a_affairs/a_affairs_002656.html Nishinomiya-Uegahara and Kobe-Sanda Warning/Strike Lifted Nishinomiya-Seiwa Campus Campus By 6:00 am All classes held as usual 1st period class cancelled By 8:00 am Both 2nd-5th period class held as usual Undergraduate 1st & 2nd period classes cancelled By 10:30 am All classes and Graduate 3rd - 5th period classes held as usual cancelled School 1st - 3rd period classes cancelled By 12:00 pm 4th - 5th period classes held as usual Any time after 12:00 pm All classes cancelled 1st - 5th period classes cancelled Graduate By 3:00 pm 6th – 7th period classes held as usual School only Any time after 3:00 pm All classes cancelled Areas Municipalities Hanshin Kobe, Amagasaki, Nishinomiya, Ashiya, Itami, Takarazuka, Kawanishi, Sanda, Inagawa Hokuban Tanba Nishiwaki, Sasayama, Tanba, Taka-cho Harima Nantobu Akashi, Kakogawa, Miki, Takasago, Ono, Kasai, Kato, Inami-cho, Harima-cho Osaka Osaka city Kita Osaka Toyonaka, Ikeda, Suita, Takatsuki, Ibaraki, Minoh, Settsu, Torimoto-cho, Toyono-cho, Nose-cho Tobu Osaka Moriguchi, Hirakata, Yao, Neyagawa, Daito, Kashiwara, Kadoma, Higashi Osaka, Shijonawate, Katano Minami Kawachi Tondabayashi, Kawachinagano, Matsubara, Habikino, Fujiidera, Osaka Sayama, Taishi-cho, Kanan-cho, Chihaya Asaka-mura Senshu Sakai, Kishiwada, Izumiotsu, Kaizuka, Izumisano, Izumi, Takaishi, Sennan, Hannan, Tadaoka-cho, Kumatori-cho, Tajiri-cho, Misaki-cho 8 September 2015 Organization for Academic Affairs Kwansei Gakuin University . -

Kawachinagano City Board of Education
Implementation Case Study GIGA School Kawachinagano City Board of Education Building High-performance GIGA School Concept Environments with Highly Reliable Cat6A UTP Cables Made by Panduit Why a wiring environment from a sole manufacturer was adopted to produce high transmission quality in the educational space over the long term Situated in the extreme southeast of Osaka Prefecture, Kawachinagano City is a regional city with a population of about 100,000, bordering the Kongo Mountain Range and Nara Prefecture to the east, and the Izumi Mountain Range and Wakayama Prefecture to the south. The municipality's territory is 70% covered in forest, with an abundance of cultural heritage sites remaining among ample natural environs. Known as a town where the Middle Ages can still be encountered, it is also the third in the nation to declare education as a key priority. Kawachinagano City made the application of ICT environments a focus of its educational effort early on, adopting tablet computers for elementary and middle school educators, installing projectors and large-screen TVs in classrooms, and offering international exchange classes using teleconferencing systems. As implementation of the GIGA School concept has been hastened amid the COVID-19 pandemic, the city has carried out an upgrade of its internal school networks, including the uniform use of Panduit products for network wiring to build infrastructure that will provide high-performance transmission into the future. As efforts toward the GIGA School concept were accelerated, the -

Voting Patterns of Osaka Prefecture
W&M ScholarWorks Dissertations, Theses, and Masters Projects Theses, Dissertations, & Master Projects 1974 The Post-War Democratization of Japan: Voting Patterns of Osaka Prefecture Hiroyuki Hamada College of William & Mary - Arts & Sciences Follow this and additional works at: https://scholarworks.wm.edu/etd Part of the Asian Studies Commons, Demography, Population, and Ecology Commons, and the Political Science Commons Recommended Citation Hamada, Hiroyuki, "The Post-War Democratization of Japan: Voting Patterns of Osaka Prefecture" (1974). Dissertations, Theses, and Masters Projects. Paper 1539624882. https://dx.doi.org/doi:10.21220/s2-yyex-rq19 This Thesis is brought to you for free and open access by the Theses, Dissertations, & Master Projects at W&M ScholarWorks. It has been accepted for inclusion in Dissertations, Theses, and Masters Projects by an authorized administrator of W&M ScholarWorks. For more information, please contact [email protected]. THE POST-WAR DEMOCRATIZATION OF JAPAN: n VOTING PATTERNS OF OSAKA PREFECTURE A Thesis Presented to The Faculty of the Department of Sociology The College of William and Mary in Virginia In Partial Fulfillment Of the Requirements of the Degree of Master of Arts by Hiroyuki Hamada May, 197^ APPROVAL SHEET This thesis is submitted in partial fulfillment of the requirements for the degree of Master of Arts Approved: May, 197^ Edwin H. Rh: Satoshi Ito, Ph.D. ___ Elaine M. The mo ^ Ph.D. DEDICATION I dedicate this thesis to my father, Kazuo Hamada, OSAKA, Japan. TABLE OF CONTENTS Page ACKNOWLEDGEMENTS ............... iv LIST OF TABLES ............... v LIST OF MAPS AND GRAPH .......... ....... vii ABSTRACT . ......... viii INTRODUCTION ...................... .......... 2 CHAPTER I. -

Goodman Takatsuki
Completion | Mid 2022 Goodman Takatsuki OVERVIEW+ ++ Located inland of Osaka, along Osaka Prefectural road 16 in the Hokusetsu area ++ A modern 4-story logistics facility with a leased area of approximately 6,600 tsubo ++ The surrounding area is densely populated and well-located for employment Driving distance Within 60 minutes driving distance Within 60 minutes Within 30 minutes Kyoto Kyoto Station Nagaokakyo Hyogo Takatsuki + Ibaraki PLANS Goodman Takatsuki Takarazuka Toyonaka Hirakata Amagasaki Nara Osaka StationOsaka A B A Higashi-Osaka Kobe Nara Port Kobe Osaka Airport Port source:Esri and Michael Bauer Research Floor 2/3 Floor 4 LOCATION+ ++ About 2 km from the JR Takatsuki Station and the Hankyu Takatsukishi Station Gross lettable area ( tsubo) ++ A bus stop is located nearby within walking distance Warehouse+ Piloti + About 5.6km from Takatsuki Interchange of Shin-Meishin Expressway, 8km from Ibaraki Interchange of Meishin Floor Office Total + berths driveway Expressway and 8km from Settsu-Kita Interchange of Kinki Expressway 4F A 620 − 40 660 ++ Good access to the Meishin and Shin-Meishin Expressways as well as to the Osaka CBD and Hokusetsu area B 1,275 − 5 1,280 3F A 605 − 130 735 hin-Meishin Expressway 2 km B 1,275 − 5 1,280 2F Takatsuki from JR Takatsuki A B A 605 − 130 735 JCT tation ankyu IC Takatsukishi B 1,050 220 10 1,280 JR Kyoto Line tation 1F Takatsuki A 420 180 50 650 Meishin B 3,600 220 20 3,840 Expressway Hankyu Kyoto Line Total Takatsukishi 5.6 km A 2,250 180 350 2,780 171 from Takatsuki IC Shin-Meishin Expy -
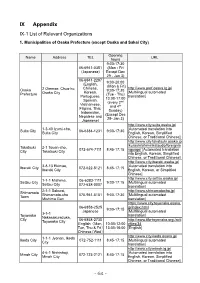
IX Appendix IX-1 List of Relevant Organizations
IX Appendix IX-1 List of Relevant Organizations 1. Municipalities of Osaka Prefecture (except Osaka and Sakai City) Opening Name Address TEL URL hours 9:00-17:30 06-6941-0351 (Mon - Fri (Japanese) Except Dec 29 - Jan 3) 06-6941-2297 9:00-20:00 (English, (Mon & Fri) Chinese, http://www.pref.osaka.lg.jp/ Osaka 2 Otemae, Chuo-ku, 9:00-17:30 Korean, [Multilingual automated Prefecture Osaka City (Tue - Thu) Portuguese, translation] 13:00-17:00 Spanish, (every 2nd Vietnamese, and 4th Filipino, Thai, Sunday) Indonesian, (Except Dec Nepalese and 29- Jan 3) Japanese) http://www.city.suita.osaka.jp/ 1-3-40 Izumi-cho, [Automated translation into Suita City 06-6384-1231 9:00-17:30 Suita City English, Korean, Simplified Chinese, or Traditional Chinese] http://www.city.takatsuki.osaka.jp /kurashi/shiminkatsudo/foreignla Takatsuki 2-1 Touen-cho, 072-674-7111 8:45-17:15 nguage/ [Automated translation City Takatsuki City into English, Korean, Simplified Chinese, or Traditional Chinese] http://www.city.ibaraki.osaka.jp/ 3-8-13 Ekimae, [Automated translation into Ibaraki City 072-622-8121 8:45-17:15 Ibaraki City English, Korean, or Simplified Chinese] http://www.city.settsu.osaka.jp/ 1-1-1 Mishima, 06-6383-1111 Settsu City 9:00-17:15 [Multilingual automated Settsu City 072-638-0007 translation] 2-1-1 Sakurai, http://www.shimamotocho.jp/ Shimamoto Shimamoto-cho 075-961-5151 9:00-17:30 [Multilingual automated Town Mishima Gun translation] https://www.city.toyonaka.osaka. 06-6858-2525 jp/index.html 9:00-17:15 Japanese [Multilingual automated 3-1-1 Toyonaka -

Membership Register MBR0009
LIONS CLUBS INTERNATIONAL CLUB MEMBERSHIP REGISTER SUMMARY THE CLUBS AND MEMBERSHIP FIGURES REFLECT CHANGES AS OF JANUARY 2021 CLUB CLUB LAST MMR FCL YR MEMBERSHI P CHANGES TOTAL DIST IDENT NBR CLUB NAME COUNTRY STATUS RPT DATE OB NEW RENST TRANS DROPS NETCG MEMBERS 5172 023732 ARIDA JAPAN 335 B 4 01-2021 80 1 0 0 -1 0 80 5172 023733 DAITO JAPAN 335 B 4 01-2021 22 0 0 0 0 0 22 5172 023734 FUJIIDERA JAPAN 335 B 4 01-2021 50 3 0 0 0 3 53 5172 023735 HIGASHI OSAKA FUSE JAPAN 335 B 4 01-2021 34 4 0 0 0 4 38 5172 023737 HIGASHI OSAKA KIKUSUI JAPAN 335 B 4 01-2021 38 2 0 1 -2 1 39 5172 023738 GOBO JAPAN 335 B 4 01-2021 42 0 0 0 -1 -1 41 5172 023739 HABIKINO JAPAN 335 B 4 01-2021 50 1 0 0 -1 0 50 5172 023740 HASHIMOTO JAPAN 335 B 4 01-2021 37 2 0 0 -2 0 37 5172 023742 HIRAKATA JAPAN 335 B 4 01-2021 145 4 0 0 -4 0 145 5172 023743 HIGASHI OSAKA JAPAN 335 B 4 01-2021 65 1 0 0 -11 -10 55 5172 023744 IBARAKI JAPAN 335 B 4 01-2021 140 6 0 0 -8 -2 138 5172 023745 IKEDA JAPAN 335 B 4 01-2021 68 1 0 0 -1 0 68 5172 023746 ITO KOYASAN L C JAPAN 335 B 4 01-2021 25 0 0 0 -1 -1 24 5172 023747 IZUMIOSAKA JAPAN 335 B 4 11-2020 34 2 0 0 -1 1 35 5172 023748 IZUMI OTSU JAPAN 335 B 4 01-2021 119 3 0 0 -3 0 119 5172 023750 IZUMISANO CHUO JAPAN 335 B 4 01-2021 46 1 0 0 -2 -1 45 5172 023752 KAINAN JAPAN 335 B 4 01-2021 33 0 0 0 0 0 33 5172 023753 KAIZUKA JAPAN 335 B 4 01-2021 34 1 0 0 0 1 35 5172 023754 KAWACHINAGANO JAPAN 335 B 4 01-2021 29 1 0 0 -2 -1 28 5172 023755 HIGASHI OSAKA KAWACHI JAPAN 335 B 4 08-2020 22 6 0 0 0 6 28 5172 023756 KASHIWARA JAPAN 335 B 4 01-2021 -

Osaka University Hospital Outline
英語版 [Access] To Saito-nishi To Saito-nishi Osaka University Hospital To Kyoto Kita-senri Hankyu Railway Handai- To Takarazuka Hankyu Railway (Takarazuka Line) (Senri Line) byoin-mae Route bus JR (Kyoto Line) Osaka Monorail Osaka Senri-chuo Bampaku- International Airport Hotarugaike kinen-koen Saito Line Ibaraki Osaka Monorail Yamada Hankyu Railway (Kyoto Line) Kita Osaka Senri-mon Osaka Airport Kyuko Railway Ibaraki-shi Esaka Minami-ibaraki Osaka University Hospital To Shin-kobe To Kyoto Zuion-ike JR Sanyo Shinkansen Nishi-mon Shin-osaka JR Tokaido Shinkansen Kita-senri Osaka Higashi-mon Hankyu Railway (Kobe Line) Dainichi University Awaji Juso Suita Campus Handai-byoin-mae To Sannomiya JR (Kobe Line) Osaka Osaka Metro Keihan Railway Sei-mon (Umeda) (Tanimachi Line) Hanshin Railway Higashi- Nishi-umeda umeda Kadoma-shi Koen-higashiguchi Osaka Noe Bampaku-gaishu Road Yodoyabashi National Museum of Metro Takaida- Osaka Metro Ethnology Kyobashi Chuo (Chuo Line) Honmachi (Sakaisuji Kawachi- Morinomiya Eiwa To Nara Expo Park Namba Suita Tower of the Sun Exit Kintetsu Railway (Nara Line) Line) Tsuruhashi Tennoji Osaka Higashi Line Kyuhoji JR (Yamatoji Line) To To JR (Osaka Loop Line) Yamada Bampaku-kinen- Kintetsu Railway Senri-chuo/Osaka-Airport koen Unobe/Kadoma-shi (Minami-osaka Line) Osaka Monorail Tengachaya (Senri Hankyu Kansai (Midosuji Osaka To Nara Chuo-kanjo-sen International Airport Osaka Metro Line) Metro (Yotsubashi Line) Railway Line) To Kashihara Chugoku Expressway Nankai Electric Railway Izumisano JR (Hanwa Line) To Wakayama