View PDF of Long-Toed Salamander
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Western Tiger Salamander,Ambystoma Mavortium
COSEWIC Assessment and Status Report on the Western Tiger Salamander Ambystoma mavortium Southern Mountain population Prairie / Boreal population in Canada Southern Mountain population – ENDANGERED Prairie / Boreal population – SPECIAL CONCERN 2012 COSEWIC status reports are working documents used in assigning the status of wildlife species suspected of being at risk. This report may be cited as follows: COSEWIC. 2012. COSEWIC assessment and status report on the Western Tiger Salamander Ambystoma mavortium in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. xv + 63 pp. (www.registrelep-sararegistry.gc.ca/default_e.cfm). Previous report(s): COSEWIC. 2001. COSEWIC assessment and status report on the tiger salamander Ambystoma tigrinum in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. vi + 33 pp. (www.sararegistry.gc.ca/status/status_e.cfm). Schock, D.M. 2001. COSEWIC assessment and status report on the tiger salamander Ambystoma tigrinum in Canada, in COSEWIC assessment and status report on the tiger salamander Ambystoma tigrinum in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. 1-33 pp. Production note: COSEWIC would like to acknowledge Arthur Whiting for writing the status report on the Western Tiger Salamander, Ambystoma mavortium, in Canada, prepared under contract with Environment Canada. This report was overseen and edited by Kristiina Ovaska, Co-chair of the COSEWIC Amphibians and Reptiles Specialist Subcommittee. For additional copies contact: COSEWIC Secretariat c/o Canadian Wildlife Service Environment Canada Ottawa, ON K1A 0H3 Tel.: 819-953-3215 Fax: 819-994-3684 E-mail: COSEWIC/[email protected] http://www.cosewic.gc.ca Également disponible en français sous le titre Ếvaluation et Rapport de situation du COSEPAC sur la Salamandre tigrée de l’Ouest (Ambystoma mavortium) au Canada. -

Successful Reproduction of the Mole Salamander Ambystoma Talpoideum in Captivity, with an Emphasis on Stimuli Environmental Determinants
SHORT NOTE The Herpetological Bulletin 141, 2017: 28-31 Successful reproduction of the mole salamander Ambystoma talpoideum in captivity, with an emphasis on stimuli environmental determinants AXEL HERNANDEZ Department of Environmental Sciences, Faculty of Sciences and Technics, University Pasquale Paoli of Corsica, Corte, 20250, France Author Email: [email protected] ABSTRACT - Generating and promoting evidence-based husbandry protocols for urodeles, commonly known as newts and salamanders, is urgently needed because most of the up-to-date ex situ programs are focused on frogs and toads than Urodela. Data on biology, life history, ecology and environmental parameters are lacking for many species and are needed to establish suitable husbandry and breeding conditions in captive environments. Two adult females and two adult males, of the mole salamander Ambystoma talpoideum successfully reproduced in captivity. It was found that reproduction of this species depends on various complex stimuli: including natural photoperiod 12:12, rainwater (acidic to neutral pH) and an aquarium full of various debris. Additionally high temperature variations ranging from 2 °C to 17 °C (a decrease followed by an increase) between November and February showed that it is possible to breed adults in aquariums provided the right stimuli are applied at the right moment of time in winter. A. talpoideum shows an explosive breeding mode as previously reported for the whole genus Ambystoma. INTRODUCTION with an emphasis on the environmental determinant stimuli involved. These data may assist in improving breeding these ince the 1980s, the current global amphibian extinction salamanders under artificial conditions. crisis has been discussed and acknowledged (Wake, A. -

Abundance, Distribution, Population Structure, and Substrate Use of Ambystoma Altamirani Along the Arroyo Los Axolotes, State of Mexico, Mexico
Herpetological Conservation and Biology 15(1):188–197. Submitted: 16 August 2019; Accepted: 23 February 2020; Published: 30 April 2020. ABUNDANCE, DISTRIBUTION, POPULATION STRUCTURE, AND SUBSTRATE USE OF AMBYSTOMA ALTAMIRANI ALONG THE ARROYO LOS AXOLOTES, STATE OF MEXICO, MEXICO VIRIDIANA VILLARREAL HERNÁNDEZ1, GEOFFREY R. SMITH2, RAYMUNDO MONTOYA AYALA3, AND JULIO A. LEMOS-ESPINAL1,4 1Laboratorio de Ecología - Unidad de Biotecnología y Prototipos, Facultad de Estudios Superiores Iztacala, Avendina Los Barrios 1, Los Reyes Iztacala, Tlalnepantla, Estado de México, 54090, México 2Department of Biology, Denison University, Granville, Ohio 43023, USA 3Laboratorio de Cómputo - Unidad de Biotecnología y Prototipos, Facultad de Estudios Superiores Iztacala, Avenida Los Barrios 1, Los Reyes Iztacala, Tlalnepantla, Estado de México, 54090, México 4Corresponding author: e-mail: [email protected] Abstract.—Ambystomatid salamanders in central Mexico are confronted by anthropogenic threats that can limit their distribution and abundance. Ambystoma altamirani (Mountain Stream Siredon) is listed as Endangered by the International Union for Conservation of Nature (IUCN) Red List and as Threatened by the Mexican government. We report on the distribution, abundance, occupancy, population structure, and substrate use of A. altamirani, a stream dwelling salamander, along the Arroyo los Axolotes, Sierra de las Cruces, Mexico. We observed A. altamirani at least once during repeated surveys between February 2018 to December 2018 in 24 of 25 permanent 5-m long reaches separated by 40 m. The best model for occupancy had constant occupancy, detection, extinction, and colonization probabilities. Sites that dried at some time during the study had fewer observed individuals than those that did not dry. Size structure was relatively constant throughout the year, except for the appearance of small larvae in May, June, and July. -

AMPHIBIA: CAUDATA: AMBYSTOMATIDAE Catalogue Of
75.1 AMPHIBIA: CAUDATA: AMBYSTOMATIDAE AMBYSTOMA Catalogue of American Amphibians and Reptiles. Acholotes: Cope, 1867:184. An incorrect subsequent spelling ofAxolotes Owen, 1844; without nomenclatural status. TIHEN,JOSEPHA. 1969. Ambystoma. Pectoglossa Mivart, 1867:698. Type-species Plethodon persimi· lis Gray, 1859 (= Salamandra jeffersoniana Green, 1827), by monotypy. A.mbystoma Salamandroides: Boulenger, 1882:38. An incorrect subsequent Mole salamanders spelling of Salamandroidis Fitzinger, 1843; without no· menclatural status. Axolotus Jarocki, 1822:179. Type-species Siren pisciformis Linguaelapsus Cope, 1887:88. Type-species Amblystoma annu• Shaw, 1802 (= Gyrinus mexicanus Shaw, 1789), by sub• latum Cope, 1886, by subsequent designation (Dunn and sequent designation (Smith and Tihen, 1961b). See No• Dunn, 1940). menclatural History. Plioambystoma Adams and Martin, 1929:17. Type-species Philhydrus Brookes, 1828:16. Type-species Siren pisciformis Plioambystoma kansense Adams and Martin, 1929, by Shaw, 1802 (= Gyrinus mexicanus Shaw, 1789), by mono• monotypy. typy. See Nomenclatural History. Bathysiredon Dunn, 1939:1. Type-species Siredon dumerilii Siredon Wagler, 1830:209, 210. Type-species Siredon axolotl Duges, 1870, by original designation. Wagler, 1830 (= Gyrinus mexicanus Shaw, 1789), by Lanebatrachus Taylor, 1941:180. Type-species Lanebatrachus monotypy. See Nomenclatural History. martini Taylor, 1941 (= Plioambystoma kansense Adams Phyllhydrus Gray, 1831:108. Type-species Siren pisciformis and Martin, 1929), by original designation. Shaw,1802 (= Gyrinus mexicanus Shaw, 1789), by mono• Ogallalabatrachus Taylor, 1941 :181. Type-species Ogallala• typy (although Gray suggested other species as possibly batrachus horarium Taylor, 1941 (= Plioambystoma kan• referable to this genus). See Nomenclatural History. sense Adams and Martin, 1929), by original designation. Axolot Bonaparte, 1831:77. Type-species Siren pisciformis Shaw, 1802 (= Gyrinus mexicanus Shaw, 1789), by im• • CONTENT. -

Recovery Strategy for the Pacific Giant Salamander (Dicamptodon Tenebrosus) in British Columbia
British Columbia Recovery Strategy Series Recovery Strategy for the Pacific Giant Salamander (Dicamptodon tenebrosus) in British Columbia Prepared by the Pacific Giant Salamander Recovery Team April 2010 About the British Columbia Recovery Strategy Series This series presents the recovery strategies that are prepared as advice to the Province of British Columbia on the general strategic approach required to recover species at risk. The Province prepares recovery strategies to meet its commitments to recover species at risk under the Accord for the Protection of Species at Risk in Canada, and the Canada – British Columbia Agreement on Species at Risk. What is recovery? Species at risk recovery is the process by which the decline of an endangered, threatened, or extirpated species is arrested or reversed, and threats are removed or reduced to improve the likelihood of a species’ persistence in the wild. What is a recovery strategy? A recovery strategy represents the best available scientific knowledge on what is required to achieve recovery of a species or ecosystem. A recovery strategy outlines what is and what is not known about a species or ecosystem; it also identifies threats to the species or ecosystem, and what should be done to mitigate those threats. Recovery strategies set recovery goals and objectives, and recommend approaches to recover the species or ecosystem. Recovery strategies are usually prepared by a recovery team with members from agencies responsible for the management of the species or ecosystem, experts from other agencies, universities, conservation groups, aboriginal groups, and stakeholder groups as appropriate. What’s next? In most cases, one or more action plan(s) will be developed to define and guide implementation of the recovery strategy. -
I Online Supplementary Data – Sexual Size Dimorphism in Salamanders
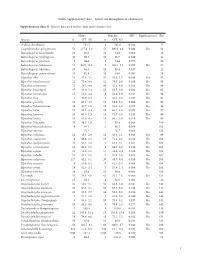
Online Supplementary data – Sexual size dimorphism in salamanders Supplementary data S1. Species data used in this study and references list. Males Females SSD Significant test Ref Species n SVL±SD n SVL±SD Andrias davidianus 2 532.5 8 383.0 -0.280 12 Cryptobranchus alleganiensis 53 277.4±5.2 52 300.9±3.4 0.084 Yes 61 Batrachuperus karlschmidti 10 80.0 10 84.8 0.060 26 Batrachuperus londongensis 20 98.6 10 96.7 -0.019 12 Batrachuperus pinchonii 5 69.6 5 74.6 0.070 26 Batrachuperus taibaiensis 11 92.9±12.1 9 102.1±7.1 0.099 Yes 27 Batrachuperus tibetanus 10 94.5 10 92.8 -0.017 12 Batrachuperus yenyuadensis 10 82.8 10 74.8 -0.096 26 Hynobius abei 24 57.8±2.1 34 55.0±1.2 -0.048 Yes 92 Hynobius amakusaensis 22 75.4±4.8 12 76.5±3.6 0.014 No 93 Hynobius arisanensis 72 54.3±4.8 40 55.2±4.8 0.016 No 94 Hynobius boulengeri 37 83.0±5.4 15 91.5±3.8 0.102 Yes 95 Hynobius formosanus 15 53.0±4.4 8 52.4±3.9 -0.011 No 94 Hynobius fuca 4 50.9±2.8 3 52.8±2.0 0.037 No 94 Hynobius glacialis 12 63.1±4.7 11 58.9±5.2 -0.066 No 94 Hynobius hidamontanus 39 47.7±1.0 15 51.3±1.2 0.075 Yes 96 Hynobius katoi 12 58.4±3.3 10 62.7±1.6 0.073 Yes 97 Hynobius kimurae 20 63.0±1.5 15 72.7±2.0 0.153 Yes 98 Hynobius leechii 70 61.6±4.5 18 66.5±5.9 0.079 Yes 99 Hynobius lichenatus 37 58.5±1.9 2 53.8 -0.080 100 Hynobius maoershanensis 4 86.1 2 80.1 -0.069 101 Hynobius naevius 72.1 76.7 0.063 102 Hynobius nebulosus 14 48.3±2.9 12 50.4±2.1 0.043 Yes 96 Hynobius osumiensis 9 68.4±3.1 15 70.2±3.0 0.026 No 103 Hynobius quelpaertensis 41 52.5±3.8 4 61.3±4.1 0.167 Yes 104 Hynobius -

Scott, DE 2005. Ambystoma Opacum (Marbled Salamander)
(Murphy, 1962) to hundreds (Graham, with males exhibiting nudging, head- B. Eggs. 1971; Shoop and Doty, 1972; Stenhouse, swinging, lifting, and body-flexing be- i. Egg deposition sites. Breeding sites are 1985a), ϳ1,000 (Pechmann et al., 1991; haviors (Arnold, 1972). Spermatophore generally the dried beds of temporary Semlitsch et al., 1996) to Ͼ10,000 (Taylor deposition follows lateral undulations of ponds, the margins of reduced ponds, or and Scott, 1997). However, given the re- the tail. Spermatophores are 4–5.5 mm tall dry floodplain pools. Female marbled liance of marbled salamanders on small (Lantz, 1930; illustrated in Noble and salamanders construct nests and lay eggs isolated seasonal wetlands and intact Brady, 1933). Typical and secondary sper- under virtually any cover in situations forested floodplain habitats, their abun- matophore deposition may occur (Arnold, where the nest is likely to be flooded by dance presumably has declined as wetland 1972, 1976; personal observation); a male subsequent winter rains (Noble and Brady, habitats have been destroyed (Petranka, may deposit over 10 spermatophores in 1933). Eggs are laid on the edges of pools 1998). For example, from the 1950s–70s 30–45 min (L. Houck, personal communi- (Dunn, 1917b) and in dry basins under the loss of wetlands in the Southeast was cation). Males will mate with females vegetation ( Jackson et al., 1989), logs greater than in any other region of the outside what is typically thought of as (Bishop, 1924; Doody, 1996), and leaf de- country, with a net annual loss of 386,000 the wetland margin (Krenz and Scott, bris (Deckert, 1916; Petranka and Petranka, ac/yr (Hefner and Brown, 1985); in North 1994). -

Key to the Identification of Streamside Salamanders
Key to the Identification of Streamside Salamanders Ambystoma spp., mole salamanders (Family Ambystomatidae) Appearance : Medium to large stocky salamanders. Large round heads with bulging eyes . Larvae are also stocky and have elaborate gills. Size: 3-8” (Total length). Spotted salamander, Ambystoma maculatum Habitat: Burrowers that spend much of their life below ground in terrestrial habitats. Some species, (e.g. marbled salamander) may be found under logs or other debris in riparian areas. All species breed in fishless isolated ponds or wetlands. Range: Statewide. Other: Five species in Georgia. This group includes some of the largest and most dramatically patterned terrestrial species. Marbled salamander, Ambystoma opacum Amphiuma spp., amphiuma (Family Amphiumidae) Appearance: Gray to black, eel-like bodies with four greatly reduced, non-functional legs (A). Size: up to 46” (Total length) Habitat: Lakes, ponds, ditches and canals, one species is found in deep pockets of mud along the Apalachicola River floodplains. A Range: Southern half of the state. Other: One species, the two-toed amphiuma ( A. means ), shown on the right, is known to occur in A. pholeter southern Georgia; a second species, ,Two-toed amphiuma, Amphiuma means may occur in extreme southwest Georgia, but has yet to be confirmed. The two-toed amphiuma (shown in photo) has two diminutive toes on each of the front limbs. Cryptobranchus alleganiensis , hellbender (Family Cryptobranchidae) Appearance: Very large, wrinkled salamander with eyes positioned laterally (A). Brown-gray in color with darker splotches Size: 12-29” (Total length) A Habitat: Large, rocky, fast-flowing streams. Often found beneath large rocks in shallow rapids. Range: Extreme northern Georgia only. -

Salamanders of the Mio-Pliocene Gray Fossil Site, Washington County, Tennessee
East Tennessee State University Digital Commons @ East Tennessee State University Electronic Theses and Dissertations Student Works 5-2009 Salamanders of the Mio-Pliocene Gray Fossil Site, Washington County, Tennessee. Grant Stanley Boardman East Tennessee State University Follow this and additional works at: https://dc.etsu.edu/etd Part of the Paleontology Commons Recommended Citation Boardman, Grant Stanley, "Salamanders of the Mio-Pliocene Gray Fossil Site, Washington County, Tennessee." (2009). Electronic Theses and Dissertations. Paper 1790. https://dc.etsu.edu/etd/1790 This Thesis - Open Access is brought to you for free and open access by the Student Works at Digital Commons @ East Tennessee State University. It has been accepted for inclusion in Electronic Theses and Dissertations by an authorized administrator of Digital Commons @ East Tennessee State University. For more information, please contact [email protected]. Salamanders of the Mio-Pliocene Gray Fossil Site, Washington County, Tennessee _____________________ A thesis presented to the faculty of the Department of Biological Sciences East Tennessee State University In partial fulfillment of the requirements for the degree Master of Science in Biological Sciences _____________________ by Grant Stanley Boardman May 2009 _____________________ Blaine W. Schubert, Chair Steven C. Wallace Thomas F. Laughlin Jim I. Mead Keywords: Mio-Pliocene, Caudata, Appalachian, Salamander ABSTRACT Salamanders of the Mio-Pliocene Gray Fossil Site, Washington County, Tennessee by Grant Stanley Boardman Screening efforts at the Gray Fossil Site, Washington County, Tennessee, have yielded a unique and diverse salamander fauna for the southern Appalachian Mio-Pliocene; including at least five taxa from three modern families (Ambystomatidae, Plethodontidae, and Salamandridae) supporting the woodland-pond interpretation of the site. -

Volume 2, Chapter 14-8: Salamander Mossy Habitats
Glime, J. M. and Boelema, W. J. 2017. Salamander Mossy Habitats. Chapt. 14-8. In: Glime, J. M. Bryophyte Ecology. Volume 2. 14-8-1 Bryological Interaction.Ebook sponsored by Michigan Technological University and the International Association of Bryologists. Last updated 19 July 2020 and available at <http://digitalcommons.mtu.edu/bryophyte-ecology2/>. CHAPTER 14-8 SALAMANDER MOSSY HABITATS Janice M. Glime and William J. Boelema TABLE OF CONTENTS Tropical Mossy Habitats – Plethodontidae........................................................................................................ 14-8-3 Terrestrial and Arboreal Adaptations ......................................................................................................... 14-8-3 Bolitoglossa (Tropical Climbing Salamanders) ......................................................................................... 14-8-4 Bolitoglossa diaphora ................................................................................................................................ 14-8-5 Bolitoglossa diminuta (Quebrada Valverde Salamander) .......................................................................... 14-8-5 Bolitoglossa hartwegi (Hartweg's Mushroomtongue Salamander) ............................................................ 14-8-5 Bolitoglossa helmrichi ............................................................................................................................... 14-8-5 Bolitoglossa jugivagans ............................................................................................................................ -

Species Accounts -- Animals
SoCal Biodiversity - Animals Arboreal Salamander Amphibian SoCal Biodiversity - Animals Arboreal Salamander Amphibian Arroyo Toad Arboreal Salamander Arboreal Salamander (Aneides lugubris) Management Status Heritage Status Rank: G5N5S4 Federal: None State: None Other: Species identified as a local viability concern (Stephenson and Calcarone 1999) General Distribution Arboreal salamander occurs in yellow pine and black oak forests in the Sierra Nevada, and in coastal live oak woodlands from northern California to Baja California. The species also occurs in the foothills of the Sierra Nevada from El Dorado County to Madera County and on South Farallon, Santa Catalina, Los Coronados, and Ano Nuevo islands off the coast of California (Petranka 1998, Stebbins 1951, Stebbins 1985). Arboreal salamander occurs from sea level to an elevation of about 5,000 feet (1,520 meters) (Stebbins 1985). Distribution in the Planning Area Arboreal salamander reportedly occurs in the foothills and lower elevations of every mountain range on National Forest System lands, although it is seldom seen (Stephenson and Calcarone 1999). There are records of occurrence for this species on the Los Padres National Forest near upper San Juan Creek and on the Cleveland National Forest near Soldier Creek (USDA Forest Service file information), San Gabriel foothills east to Day Canyon, and in the San Jacinto Mountains (Goodward pers. comm.). Systematics There are four species in the genus Aneides in the western United States, three of which occur in California (Stebbins 1985). Of these three, only arboreal salamander ranges into southern California. Most of the Aneides salamanders climb (Stebbins 1985). Arboreal salamander consists of two chromosomally differentiated groups that intergrade in south and east-central Mendocino County, about 56 miles (90 kilometers) north of the San Francisco Bay region (Sessions and Kezer 1987). -

Comparative Anatomy and Phylogeny of the Cloacae of Salamanders (Amphibia: Caudata)
JOURNAL OF MORPHOLOGY 212~305-322 (1992) Comparative Anatomy and Phylogeny of the Cloacae of Salamanders (Amphibia: Caudata). Vi. Ambystomatidae and Dicamptodontidae DAVID M. SEVER Department of Biology, Saint Mary's College, Notre Dame, Indiana 46556 ABSTRACT Histology of the cloacae of Rhyacotriton olympicus and represen- tative species from the genera Ambystoma and Dicamptodon was examined by light microscopy. Females of Ambystoma possess sperm storage glands, the spermathecae, as well as ventral glands and dorsal glands, both of uncertain function. Females of Ambystoma examined from the subgenus Linguaelapsus differ from those in the subgenus Ambystoma by possessing more extensive ventral gland clusters and a shorter cloacal tube. Females of Dicamptodon possess spermathecae and ventral glands, but differ in cloacal conformation from females of Ambystoma and lack the dorsal glands. Females of R. olympi- cus possess more extensive epidermal lining in the cloaca than that found in females of Ambystoma and Dicarnptodon, and the only glands present are spermathecae, which cluster around a tube in the dorsal roof. Males of Ambystoma, Dicamptodon, and R. olympicus possess five types of cloacal glands (dorsal pelvic glands, lateral pelvic glands, anterior ventral glands, posterior ventral glands, and Kingsbury's glands) that function in spermato- phore formation, and vent glands that may produce a courtship pheromone. In Ambystoma and Dicamptodon, vent glands secrete along the medial borders of the cloacal orifice. Males of A. opacum and A. talpoideum differ from males of other species examined from the two genera by possessing more extensive vent glands. Males of R. olympicus possess unique vent glands in which tubules secrete onto the surface of vent lobes lateral to the posterior end of the cloacal orifice, and distal ends of the glands pass anteriorly, superficial to the fascia enclosing the other cloacal glands.