C:\Documents and Settings\Alan Smithee\My Documents\MOTM\Fluorite3.Wpd
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Anroach Farm We Hope You’Ll Love It As Much As We Do
An roach Far m Room guide LOVE IT HERE Welcome to Anroach Farm We hope you’ll love it as much as we do... Windows and Doors If you leave a ground floor window or the door open you may end up meeting one of our friendly cats - ‘Pebbles’ and ‘Henry’. Keeping you Toastie The heater is on a schedule and should be warm enough. To adjust the temperature you can use the up and down arrows on the top right. If you would like to turn the heater off there is a on/off button on the right hand side of the heater. Please do not hang anything on the electric heater as this is a fire risk. What’s on the Box To turn on the Television press the on button situated on the white television remote. Use the top left button on the black remote control to turn on the free sat box and use the guide button to select the programs. There are also radio stations available on the free sat box. There is an in built DVD Player in the TV - it is to the right side of the TV. We have a selection of DVDs at the top of the stairs. If you cannot get the TV to work please check the input source on the TV is set to one of the HDMI inputs. Getting on the Line Can you do without the internet... go on we dare you! If you can’t then you can connect to ‘Anroach Wifi’ (there is no password). -

7-Night Peak District Self-Guided Walking Holiday
7-Night Peak District Self-Guided Walking Holiday Tour Style: Self-Guided Walking Destinations: Peak District & England Trip code: DVPOA-7 1, 2 & 3 HOLIDAY OVERVIEW Enjoy a break in the Peak District with the walking experts; we have all the ingredients for your perfect Self- Guided Walking holiday. Our 3-star country house, just a few minutes' walk from the limestone gorge of Dove Dale, is geared to the needs of walkers and outdoor enthusiasts. Enjoy hearty local food, detailed route notes, and an inspirational location from which to explore the stunning landscapes of the Derbyshire Dales. HOLIDAYS HIGHLIGHTS • Use our Discovery Point, stocked with maps and walks directions for exploring the local area • Head out on any of our walks to discover the varied beauty of the Peak District on foot • Enjoy panoramic views from gritstone edges • Admire stunning limestone dales • Visit classic viewpoints, timeless villages and secret corners • Look out for wildlife and learn about the 'Peaks' history • Choose a relaxed pace of discovery where you can get some fresh air in one of England's finest walking www.hfholidays.co.uk PAGE 1 [email protected] Tel: +44(0) 20 3974 8865 areas • Cycle along the nearby Tissington Trail • Discover Chatsworth House • Visit the Alton Towers theme park TRIP SUITABILITY Explore at your own pace and choose the best walk for your pace and ability. ACCOMMODATION The Peveril Of The Peak The Peveril of the Peak, named after Sir Walter Scott’s novel, stands proudly in the Peak District countryside, close to the village of Thorpe. -

Abstract Spectroscopic Characterization of Fluorite
ABSTRACT SPECTROSCOPIC CHARACTERIZATION OF FLUORITE: RELATIONSHIPS BETWEEN TRACE ELEMENT ZONING, DEFECTS AND COLOR By Carrie Wright This thesis consists of two separate papers on color in fluorite. In the first paper, synthetic fluorites doped with various REEs (10-300 ppm) were analyzed using direct current plasma spectrometry, optical absorption spectroscopy, fluorescence spectrophotometry, and electron paramagnetic resonance spectroscopy before and after receiving 10-25 Mrad of 60Co gamma irradiation. The combined results of these techniques indicate that the irradiation-induced color of the Y-, Gd-, La- and Ce-doped samples are the result of a REE-associated fluorine vacancy that traps two electrons. Divalent samarium may be the cause of the irradiation-induced green color of the Sm- doped sample. In the second paper, fluorite crystals from Bingham, NM, Long Lake, NY, and Westmoreland, NH were similarly investigated to determine the relationship between sectorally zoned trace elements, defects, and color. The results indicate causes of color similar to those in the synthetic samples with the addition of simple F-centers. SPECTROSCOPIC CHARACTERIZATION OF FLUORITE: RELATIONSHIPS BETWEEN TRACE ELEMENT ZONING, DEFECTS AND COLOR A Thesis Submitted to the Faculty of Miami University In partial fulfillment of The requirements for the degree of Master of Science Department of Geology By Carrie Wright Miami University Oxford, OH 2002 Advisor_____________________ Dr. John Rakovan Reader______________________ Dr. Hailiang Dong TABLE OF CONTENTS Chapter 1: Introduction to the cause of color in fluorite 1 Manuscript 1-Chapter 2 29 “Spectroscopic investigation of lanthanide doped CaF2 crystals: implications for the cause of color” Manuscript 2-Chapter 3 95 “Spectroscopic characterization of fluorite from Bingham, NM, Long Lake, NY and Westmoreland, NH: relationships between trace element zoning, defects and color ii TABLE OF FIGURES Chapter 1 Figures 21 Figure 1a. -
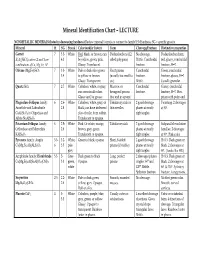
Mineral Identification Chart – LECTURE
Mineral Identification Chart – LECTURE NONMETALLIC MINERALS (listed in decreasing hardness) Review mineral formula to connect to family! H=Hardness; SG = specific gravity Mineral H SG Streak Color (and/or luster) Form Cleavage/Fracture Distinctive properties Garnet 7 3.5- White Red, black, or brown; can Dodecahedrons (12- No cleavage. Dodecahedron form, X3Y2(SiO4)3 where X and Y are 4.3 be yellow, green, pink. sided polygons) Brittle. Conchoidal red, glassy, conchoidal combinations of Ca, Mg, Fe, Al Glassy. Translucent. fracture. fracture, H=7. Olivine (Mg,Fe)2SiO4 7 3.3- White Pale or dark olive green Short prisms Conchoidal Green, conchoidal 3.4 to yellow or brown. (usually too small to fracture. fracture, glassy, H=7. Glassy. Transparent. see). Brittle. Usually granular. Quartz SiO2 7 2.7 White Colorless, white, or gray; Massive; or Conchoidal Glassy, conchoidal can occur in all colors. hexagonal prisms fracture. fracture, H=7. Hex. Glassy and/or greasy. that end in a point. prism with point end. Plagioclase Feldspar family: 6 2.6- White Colorless, white, gray, or Tabular crystals or 2 good cleavage Twinning. 2 cleavages Anorthite and Labradorite 2.8 black; can have iridescent thin needles planes at nearly at 90°. CaAl2Si2O8 to Oligoclase and play of color from within. right angles. Albite NaAlSi3O8 Translucent to opaque. Potassium Feldspar family: 6 2.5- White Pink. Or white, orange, Tabular crystals 2 good cleavage Subparallel exsolution Orthoclase and Microcline 2.6 brown, gray, green. planes at nearly lamellae. 2 cleavages KAlSi3O8 Translucent to opaque. right angles. at 90°. Pink color. Pyroxene family: Augite 5.5- 3.2- White, Green to black; opaque. -

Welcome to Buxton Caravan Club Site
Welcome to Buxton Caravan Club Site Get to know Buxton Hidden away on the valley floor, Grin Low is conveniently placed for just about everything going on in and around the Peak District, but particularly for the civilised little town of Buxton with its colourful Pavilion Gardens and the Opera House, which offers a wide range of events and the world famous Festival from mid-July to August. You’re surrounded by the Peak District National Park, which has an extensive network of cycleways, all way-marked for pleasurable exploration. If you’re looking for less energetic pursuits, there is a full set of splendid stately homes to visit. During your travels, you’ll trip over ancient customs, and you could have an interesting holiday tracking a few down, such as the beautiful floral Well Dressing, said to be a thanksgiving for water. Things to see and do from this Club Site Local attractions • Poole’s Cavern & Buxton Country Park • Chatsworth House Cascading water and incredible crystal formations combine to create One of Britain’s best loved historic houses and estates, offering the most spectacular cavern in the Peak District. something for everyone, from famous works of art and the Concessions for Club Members. spectacular fountains in the garden to the finest shopping, food & 01298 26978 drink and many miles of free walks. www.poolescavern.co.uk 01246 582204 • Churnet Valley Railway www.chatsworth.org A truly beautiful heritage railway deep in the heart of the • Haddon Hall Staffordshire Moorlands. Step back in time with a journey on a steam This medieval and Tudor manor house is an absolute gem. -

The Ultimate Peak District & Derbyshire Bucket List
The Ultimate Peak District & Derbyshire Bucket List: 101 Great Things To Do 1. Embrace the great outdoors in the UK’s first National Park Established in 1951, the Peak District is the country’s oldest National Park. If you love the outdoors, this protected area of natural beauty - which covers 555 square miles in total - offers over 200 square miles of stunning open access land to explore. 2. Visit the ‘jewel in the Peak District’s crown’ at Chatsworth House Home to the Duke and Duchess of Devonshire, Chatsworth is one of the UK’s favourite stately homes. Discover over 30 magnificent rooms, a 105-acre garden, parkland, a farmyard and playground, and one of Britain’s best farm shops. 3. Conquer the tallest ‘Peak’ in the Peak District At 636 metres above sea level, you’ll feel like you’re standing on top of the world when you conquer the Kinder Scout plateau. It’s the highest point in the National Park and was also the site of the 1932 Mass Trespass, a landmark event which sparked a debate about the right to roam in the countryside, leading to the establishment of the Peak District as the first National Park two decades later. 4. Discover the UK’s oldest Ice Age cave art at Creswell Crags Walk in the footsteps of Ice Age hunters, uncover the secrets of early man, discover incredible Ice Age cave art and marvel at the UK’s largest discovery of ritual protection marks at this picturesque limestone gorge on the Derbyshire/Nottinghamshire border. 5. -

Winter 1998 Gems & Gemology
WINTER 1998 VOLUME 34 NO. 4 TABLE OF CONTENTS 243 LETTERS FEATURE ARTICLES 246 Characterizing Natural-Color Type IIb Blue Diamonds John M. King, Thomas M. Moses, James E. Shigley, Christopher M. Welbourn, Simon C. Lawson, and Martin Cooper pg. 247 270 Fingerprinting of Two Diamonds Cut from the Same Rough Ichiro Sunagawa, Toshikazu Yasuda, and Hideaki Fukushima NOTES AND NEW TECHNIQUES 281 Barite Inclusions in Fluorite John I. Koivula and Shane Elen pg. 271 REGULAR FEATURES 284 Gem Trade Lab Notes 290 Gem News 303 Book Reviews 306 Gemological Abstracts 314 1998 Index pg. 281 pg. 298 ABOUT THE COVER: Blue diamonds are among the rarest and most highly valued of gemstones. The lead article in this issue examines the history, sources, and gemological characteristics of these diamonds, as well as their distinctive color appearance. Rela- tionships between their color, clarity, and other properties were derived from hundreds of samples—including such famous blue diamonds as the Hope and the Blue Heart (or Unzue Blue)—that were studied at the GIA Gem Trade Laboratory over the past several years. The diamonds shown here range from 0.69 to 2.03 ct. Photo © Harold & Erica Van Pelt––Photographers, Los Angeles, California. Color separations for Gems & Gemology are by Pacific Color, Carlsbad, California. Printing is by Fry Communications, Inc., Mechanicsburg, Pennsylvania. © 1998 Gemological Institute of America All rights reserved. ISSN 0016-626X GIA “Cut” Report Flawed? The long-awaited GIA report on the ray-tracing analysis of round brilliant diamonds appeared in the Fall 1998 Gems & Gemology (“Modeling the Appearance of the Round Brilliant Cut Diamond: An Analysis of Brilliance,” by T. -

Hope to Hathersage Or Bamford Via Castleton
Hope to Hathersage (via Castleton) Hope to Bamford (via Castleton) 1st walk check 2nd walk check 3rd walk check 1st walk check 2nd walk check 3rd walk check 17th August 2020 Current status Document last updated Wednesday, 19th August 2020 This document and information herein are copyrighted to Saturday Walkers’ Club. If you are interested in printing or displaying any of this material, Saturday Walkers’ Club grants permission to use, copy, and distribute this document delivered from this World Wide Web server with the following conditions: • The document will not be edited or abridged, and the material will be produced exactly as it appears. Modification of the material or use of it for any other purpose is a violation of our copyright and other proprietary rights. • Reproduction of this document is for free distribution and will not be sold. • This permission is granted for a one-time distribution. • All copies, links, or pages of the documents must carry the following copyright notice and this permission notice: Saturday Walkers’ Club, Copyright © 2019-2020, used with permission. All rights reserved. www.walkingclub.org.uk This walk has been checked as noted above, however the publisher cannot accept responsibility for any problems encountered by readers. Hope to Hathersage or Bamford (via Castleton) Start: Hope Station Finish: Hathersage or Bamford Stations Hope Station, map reference SK 180 832, is 18 km south west of Sheffield, 231 km north west of Charing Cross and 169m above sea level. Bamford Station, map reference SK 207 825, is 3 km south east of Hope Station and 151m above sea level. -

Mineralogy and Chemistry of Rare Earth Elements in Alkaline Ultramafic Rocks and Fluorite in the Western Kentucky Fluorspar District Warren H
Mineralogy and Chemistry of Rare Earth Elements in Alkaline Ultramafic Rocks and Fluorite in the Western Kentucky Fluorspar District Warren H. Anderson Report of Investigations 8 doi.org/10.13023/kgs.ri08.13 Series XIII, 2019 Cover Photo: Various alkaline ultramafic rocks showing porphyritic, brecciated, and aphanitic textures, in contact with host limestone and altered dike texture. From left to right: • Davidson North dike, Davidson core, YH-04, 800 ft depth. Lamprophyre with calcite veins, containing abundant rutile. • Coefield area, Billiton Minner core BMN 3. Intrusive breccia with lamprophyric (al- nöite) matrix. • Maple Lake area, core ML-1, 416 ft depth. Lamprophyre intrusive. • Maple Lake area, core ML-2, 513 ft depth. Lamprophyre (bottom) in contact with host limestone (top). Kentucky Geological Survey University of Kentucky, Lexington Mineralogy and Chemistry of Rare Earth Elements in Alkaline Ultramafic Rocks and Fluorite in the Western Kentucky Fluorspar District Warren H. Anderson Report of Investigations 8 doi.org/10.13023/kgs.ri08.13 Series XIII, 2019 Our Mission The Kentucky Geological Survey is a state-supported research center and public resource within the University of Kentucky. Our mission is to sup- port sustainable prosperity of the commonwealth, the vitality of its flagship university, and the welfare of its people. We do this by conducting research and providing unbiased information about geologic resources, environmen- tal issues, and natural hazards affecting Kentucky. Earth Resources—Our Common Wealth www.uky.edu/kgs © 2019 University of Kentucky For further information contact: Technology Transfer Officer Kentucky Geological Survey 228 Mining and Mineral Resources Building University of Kentucky Lexington, KY 40506-0107 Technical Level General Intermediate Technical Statement of Benefit to Kentucky Rare earth elements are used in many applications in modern society, from cellphones to smart weapons systems. -

Andradite in Andradite Unusual Growth Zoning in Beryl
Editor Nathan Renfro Contributing Editors Elise A. Skalwold and John I. Koivula Andradite in Andradite ity, but size was not what made it special. As shown in fig- Recently we had the opportunity to examine a dramatic ure 1, close examination of one of the polished crystal faces iridescent andradite fashioned by Falk Burger (Hard Works, revealed a bright reddish orange “hot spot” in the center, Tucson, Arizona) from a crystal originating from the caused by an iridescent inclusion of andradite with a dif- Tenkawa area of Nara Prefecture in Japan. Known as “rain- ferent crystallographic orientation than its host. As seen bow” andradite, this material was previously reported in in figure 2, the inclusion’s different orientation caused the iridescence of the rhomb-shaped “hot spot” to appear and Gems & Gemology (T. Hainschwang and F. Notari, “The cause of iridescence in rainbow andradite from Nara, disappear as the light source was passed over the crystal’s Japan,” Winter 2006, pp. 248–258). The specimen was surface. To see the iridescence from both the host and in- unique for its genesis and optical phenomenon. clusion at the same time, two light sources from opposite Weighing 16.79 ct and measuring 15.41 × 13.86 × 10.49 directions must be used due to the different crystallo- mm, the andradite was very large for its species and local- graphic orientation of the host and inclusion. This elusive optical phenomenon made this Japanese andradite crystal extremely interesting for any aspiring inclusionist. John I. Koivula Figure 1. This 16.79 ct Japanese andradite garnet GIA, Carlsbad exhibits a very unusual rhomb-shaped “hot spot” below the surface of one crystal face. -

Low Temperature Aqueous Solubility of Fluorite at Temperatures of 5, 25, and 50 Oc and Ionic Strengths up to 0.72M
LOW TEMPERATURE AQUEOUS SOLUBILITY OF FLUORITE AT TEMPERATURES OF 5, 25, AND 50 OC AND IONIC STRENGTHS UP TO 0.72M by Richard L. Henry A thesis submitted to the Faculty and Board of Trustees of the Colorado School of Mines in partial fulfillment of the requirement for the degree of Doctor of Philosophy (Geochemistry) Golden, Colorado Date __________________________ Signed: ______________________________ Richard L. Henry Signed: ______________________________ Dr. Wendy J. Harrison Thesis Advisor Golden, Colorado Date __________________________ Signed: ______________________________ Dr. M. Stephen Enders Professor and Head Department of Geology and Geological Engineering ii ABSTRACT Historical fluorite solubility research found that fluorite solubilities varied over three orders-of-magnitude and appear to be inconsistent with several recently determined fluorite solubilities. This research involved a comprehensive series of solubility experiments run over a period of three years using nine natural fluorites and one synthetic fluorite to determine their solubility in low temperature (5, 25, and 50oC) aqueous solutions and ionic strengths ranging from near zero to 0.72M. Equilibrium was approached from both undersaturated and supersaturated initial conditions. The fluorites were chemically and physically characterized using laboratory analyses, thin section petrography, UV fluorescence, and x-ray diffraction. The fluorite samples were generally found to have similar chemical and physical properties. The chemical composition was approximately stoichiometric, with a mean calcium concentration of 51.6 ± 0.6 wt % and mean fluorine concentration of 50.2 ± 0.7 wt %. Fluorite unit cell edge lengths, volumes, and densities averaged 5.4630 ± 0.0007 Å, 163.04 ± 0.06 Å3, and 3.181 ± 0.009 g/cm3, respectively. -

FLUORITE Var
Mineral of the Month July 2014 FLUORITE var. “RAINBOW” This month our featured mineral is the beautiful “rainbow” variety of fluorite from China. Our write-up explains the origin of its multicolored bands and discusses the development of X-ray diffraction and its importance in mineralogy and other sciences. OVERVIEW PHYSICAL PROPERTIES Chemistry: Calcium Fluoride CaF2 Class: Halides Group: Fluorite Crystal System: Isometric (Cubic) Crystal Habits: Usually cubic, often as penetration twins; less frequently octahedral; rarely dodecahedral; also occurs in botryoidal, granular, massive, columnar, and earthy forms, and as cleavage masses. Color: White, colorless, violet, purple, lilac, blue, green, yellow, brown, amber, bluish- black, pink, and rose-red; “rainbow” varieties are multicolored. Luster: Vitreous Transparency: Transparent and translucent to nearly opaque Streak: White Cleavage: Perfect in four directions, forming octahedrons. Fracture: Uneven, brittle. Hardness: 4.0 Specific Gravity: 3.0-3.2 Luminescence: Often fluorescent and phosphorescent, sometimes thermoluminescent and triboluminescent. Refractive Index: 1.433 Distinctive Features and Tests: Best field indicators are cubic crystal form and well- developed crystals; perfect, four-directional cleavage; relative softness; and occurrence in fluorine-rich, mineral environments. Dana Classification Number: 9.2.1.1 NAME: The word “fluorite,” pronounced FLOR-ite, stems from the Latin fluere, meaning “flow” and alluding to fluorite’s ability to reduce the melting temperatures of metals in smelting processes. Alternative names include “androdamant,” “bruiachite,” “Derbyshire spar,” “fluor,” “fluores,” “fluoride of calcium,” “fluoride of lime,” “fluorspar,” “fluorspath,” “flusse,” “flusspat,” “liparite,” “murrhina,” and “spath vitreux.” Color-related variety names are “false emerald,” “false amethyst,” “false ruby,” and “fluorite rose.” “Rainbow” fluorite has multicolored banding.