Handling Precautions for Viton™ and Related Chemicals
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Restriction of Per- and Polyfluoroalkyl Substances (PFAS) Under REACH
Webinar: Restriction of per- and polyfluoroalkyl substances (PFAS) under REACH Questions and answers ECHA organised a webinar on 29 October 2020 on Restriction of per- and polyfluoroalkyl substances (PFAS) under REACH. It explained the REACH restriction process and the status of ongoing work by the Netherlands, Germany, Denmark, Sweden and Norway on a potential restriction to limit the risks to the environment and human health from the manufacture and use of per- and polyfluoroalkyl substances (PFAS). This document compiles the questions and answers from the webinar. Editorial changes have been made to improve clarity, correct spelling mistakes etc. Similar questions have been combined. The document will not be updated. For the most up-to-date advice on PFAS, contact us or refer to our support material. Disclaimer: The European Chemicals Agency and the authorities from the five countries does not accept any liability with regard to the use that may be made of the information contained in this document. Use of the information in this document remains the sole responsibility of the reader. Although, the information provided on this Q&A document has been prepared with the utmost care, possible errors or omissions cannot be excluded. The European Chemicals Agency and the authorities from the five countries does not accept any liability with regards to any such errors or omissions. P.O. Box 400, FI-00121 Helsinki, Finland | Tel. +358 9 686180 | echa.europa.eu Question Answer General process We expect to notify the proposal in ECHA's registry of intention (RoI) in the first half of 2021 and to submit the completed assessment and proposal to ECHA for opinion making in the first half of 2022. -

FKM Xplor™ V9T82
TRELLEBORG SEALING SOLUTIONS FKM XploRTM V9T82 EXPLOSIVE DECOMPRESSION RESISTANT MATERIALS OUTSTANDING EXTREME LOW TEMPERATURE PERFORMANCE Explosive decompression is a major concern to the oil and gas industry. It occurs when applied system pressure is released, causing absorbed gas to expand, potentially damaging elastomer seals. Trelleborg Sealing Solutions has focused on this issue and Features and benefits presents the XploR™ range, an entire family of advanced elastomers especially developed for oil and gas applications. • Unrivalled ED restistance within its material type The portfolio includes compounds in HNBR, FKM, Aflas® and • Operating temperatures from -48°C to +200°C/-54°F to Isolast® Perfluoroelastomer, each of which demonstrates +392°F with short excursions to 210°C/+410°F best-in-class Explosive Decompression (ED) resistance for • Outstanding performance at extremely low temperatures its material type. • Exceptional mechanical performance • Low long-term compression set XploR™ V9T82 combines excellent chemical and thermal • Very good chemical compatibility properties, with outstanding low temperature capability. • Extended life in aggressive media, including the hydrocarbon It exhibits superior high pressure sealing performance in and aqueous media common within oil & gas applications ED situations, which is supported by independent institute • High modulus and high strength approval to standard test protocols. Applications When the composition of the well or conditions of the application are known, FKM XploR™ V9T82 may prove the • Separation equipment optimum and most cost-effective material for your application, • Connector systems especially when operating temperatures are extremely low. • Valves • Wellhead control equipment For further information on selecting the right compound and • Tubing hangers advice on seal specification for your individual application, • Swivel stacks on Floating Production Storage and consult your local Trelleborg Sealing Solutions marketing Offloading (FPSO) vessels company. -

Comprehensive Testing to Measure the Jtespoi^Se^Yf Jhuorocarbdn Rubber (FKM) Whanfomtank1 Waste Simulant
2t 2000 SANDIA REPORT SAND2000-032SA 5 i Unlimited Release Rrinted February 2000 Comprehensive Testing to Measure the jtespoi^se^yf JHuorocarbdn Rubber (FKM) WHanfoMTank1 Waste Simulant Paul J. Nigrey and DenniS'L. Bolton Prepared by Sandia National Laboratories Albuquerque, New Mexico 87185 and Livermore, California 94550 Sandia'is a multiprogram laboratory operated by Sandia Corporation, a Lock'heed Martin Company, for the United States Department of Energy under Contract DE-AC04-94AL85000. Approved for public release; further dissemination unlimited. Sandia National Laboratories Issued by Sandia National Laboratories, operated for the United States Department of Energy by Sandia Corporation. NOTICE: This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government, nor any agency thereof, nor any of their employees, nor any of their contractors, subcontractors, or their employees, make any warranty, express or implied, or assume any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represent that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise, does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government, any agency thereof, or any of their contractors or subcontractors. The views and opinions expressed herein do not necessarily state or reflect those of the United States Government, any agency thereof, or any of their contractors. Printed in the United States of America. This report has been reproduced directly from the best available copy. -

Xyfluor Chemical Compatibility Guide
456456 Xyfluor® Chemical Compatibility Xyfluor® is a proprietary, highly fluorinated elastomer. The oxygen in the polymer backbone provides outstanding low-temperature capabilities - far better than FKM or FFKM elastomers. The polymer provides improved resistance to many harsh chemicals that can attack the hydrogen in FKM elastomers. The chemical resistance of Xyfluor® approaches but is not equivalent to FFKM elastomers. A = Swell < 10% after exposure. Suitable. B = Swell > 10% & < 20% after exposure. Generally suitable. C = Swell >20% & < 40% after exposure. May be suitable in some situations. D = Swell > 40% after exposure. Not suitable. N = Insufficient data. Test: Full immersion, Room Temperature, 3 days The information contained herein is believed to be reliable, but no representation, guarantees or warranties of any kind are made to its accuracy or suitability for any purpose. Full-scale testing and end-product performance are the responsibility of the user. CHEMICAL RATING CHEMICAL RATING acetaldehyde A amomnium phosphate A acetic acid, ammonium stearate A glacial A ammonium sulfate A hot A ammonium thiocyanate A 5% A amyl acetate A/B acetic anhydride A amyl alcohol A acetone A amyl nitrate A acetone cyanohydrin A aniline A acetyl chloride A aniline hydrochloride A acetylene gas A anti-freeze, alcohol or glycol based A acrylonitrile A aqua regia N adipic acid A argon gas A alcohol, denatured A arsenic acid A alkyl benzene A ash slurry A alkyl-arylsulphonic acid A asphalt A alumina trihydrate N barium chloride A aluminum acetate -

Hydrogen Peroxide Material Compatibility Chart from ISM and IS
ver 09-Jul-2020 Hydrogen Peroxide Material Compatibility Chart All wetted surfaces should be made of materials that are compatible with hydrogen peroxide. The wetted area or surface of a part, component, vessel or piping is a surface which is in permanent contact with or is permanently exposed to the process fluid (liquid or gas). Less than 8% concentration H2O2 is considered a non-hazardous substance. Typically encountered versions are baking soda-peroxide toothpaste (0.5%), contact lens sterilizer (2%), over-the-counter drug store Hydrogen Peroxide (3%), liquid detergent non-chlorine bleach (5%) and hair bleach (7.5%). At 8% to 28% H2O2 is rated as a Class 1 Oxidizer. At these concentrations H2O2 is usually encountered as a swimming pool chemical used for pool shock treatments. In the range of 28.1% to 52% concentrations, H2O2 is rated as a Class 2 Oxidizer, a Corrosive and a Class 1 Unstable (reactive) substance. At these concentrations, H2O2 is considered industrial strength grade. Concentrations from 52.1% to 91% are rated as Class 3 Oxidizers, Corrosive and Class 3 Unstable (reactive) substances. H2O2 at these concentrations are used for specialty chemical processes. At concentrations above 70%, H2O2 is usually designated as high-test peroxide (HTP). Concentrations of H2O2 greater than 91% are currently used as rocket propellant. At these concentrations, H2O2 is rated as a Class 4 Oxidizer, Corrosive and a Class 3 Unstable (reactive) substance. Compatibility Compatibility Compatibility Compatibility Material 10% H2O2 30% H2O2 50% -

EPDM & FKM Chemical Resistance Guide
EPDM & FKM Chemical Resistance Guide SECOND EDITION EPDM & FKM CHEMICAL RESISTANCE GUIDE Elastomers: Ethylene Propylene (EPDM) Fluorocarbon (FKM) Chemical Resistance Guide Ethylene Propylene (EPDM) & Fluorocarbon (FKM) 2nd Edition © 2020 by IPEX. All rights reserved. No part of this book may be used or reproduced in any manner whatsoever without prior written permission. For information contact: IPEX, Marketing, 1425 North Service Road East, Oakville, Ontario, Canada, L6H 1A7 ABOUT IPEX At IPEX, we have been manufacturing non-metallic pipe and fittings since 1951. We formulate our own compounds and maintain strict quality control during production. Our products are made available for customers thanks to a network of regional stocking locations from coast-to-coast. We offer a wide variety of systems including complete lines of piping, fittings, valves and custom-fabricated items. More importantly, we are committed to meeting our customers’ needs. As a leader in the plastic piping industry, IPEX continually develops new products, modernizes manufacturing facilities and acquires innovative process technology. In addition, our staff take pride in their work, making available to customers their extensive thermoplastic knowledge and field experience. IPEX personnel are committed to improving the safety, reliability and performance of thermoplastic materials. We are involved in several standards committees and are members of and/or comply with the organizations listed on this page. For specific details about any IPEX product, contact our customer service department. INTRODUCTION Elastomers have outstanding resistance to a wide range of chemical reagents. Selecting the correct elastomer for an application will depend on the chemical resistance, temperature and mechanical properties needed. Resistance is a function both of temperatures and concentration, and there are many reagents which can be handled for limited temperature ranges and concentrations. -

New Developments in Properties for Ethylene Acrylate Polymers
18 Rubber & Plastics News • November 30, 2020 www.rubbernews.com www.rubbernews.com Technical Technical New developments in properties for ethylene acrylate polymers By Patricia Panne, Klaus Kammerer, Serge Bouvier, Ed McBride, Mark Stewart, Steve Oriani and Elias Wang Executive summary DuPont Vamac-brand ethylene acrylic elastomer (AEM) was introduced in 1975 and has been used successfully for many years in the Ethylene acrylate (AEM) polymers transportation sector, where excellent resistance to heat, engine fluids and low temperature flexibility is required. were first introduced to the elastomer With the introduction of the Vamac Ultra grades, a step-change improvement in processability, performance and customer value market more than 40 years ago. They for targeted applications has been achieved. Increasing demands for improved temperature stability led recently to the development are non-crystalline copolymers of eth- of the VMX5000 series of pre-compounds to offer compounds in applications with a 10-15°C higher heat resistance over conventional ylene and methyl acrylate. carbon black-filled AEM compounds. Resistance to engine oils and acids remains excellent, which makes the VMX5000 pre-com- Both monomers add to the high tem- pounds an interesting cost-effective alternative to FKM materials in the temperature range between -35°C and +175°C. perature stability, and the completely sat- For dynamic hose applications, slow-curing polymers (Vamac GXF, Ultra HT and HT-OR) have been introduced. While com- urated polymer chain imparts excellent pounds using the Ultra grades offer better physical properties, they sometimes lead to high extrusion pressure and therefore re- resistance to ozone, oxidation and weath- quire lower hardness compounds (<70 ShA). -

EPDM & FKM Chemical Resistance Guide
EPDM & FKM Chemical Resistance Guide SECOND EDITION EPDM & FKM CHEMICAL RESISTANCE GUIDE Elastomers: Ethylene Propylene (EPDM) Fluorocarbon (FKM) Chemical Resistance Guide Ethylene Propylene (EPDM) & Fluorocarbon (FKM) 2nd Edition © 2020 by IPEX. All rights reserved. No part of this book may be used or reproduced in any manner whatsoever without prior written permission. For information contact: IPEX, Marketing, 1425 North Service Road East, Oakville, Ontario, Canada, L6H 1A7 ABOUT IPEX At IPEX, we have been manufacturing non-metallic pipe and fittings since 1951. We formulate our own compounds and maintain strict quality control during production. Our products are made available for customers thanks to a network of regional stocking locations from coast-to-coast. We offer a wide variety of systems including complete lines of piping, fittings, valves and custom-fabricated items. More importantly, we are committed to meeting our customers’ needs. As a leader in the plastic piping industry, IPEX continually develops new products, modernizes manufacturing facilities and acquires innovative process technology. In addition, our staff take pride in their work, making available to customers their extensive thermoplastic knowledge and field experience. IPEX personnel are committed to improving the safety, reliability and performance of thermoplastic materials. We are involved in several standards committees and are members of and/or comply with the organizations listed on this page. For specific details about any IPEX product, contact our customer service department. INTRODUCTION Elastomers have outstanding resistance to a wide range of chemical reagents. Selecting the correct elastomer for an application will depend on the chemical resistance, temperature and mechanical properties needed. Resistance is a function both of temperatures and concentration, and there are many reagents which can be handled for limited temperature ranges and concentrations. -

Eriks.Be SEALING ELEMENTS
sealing elements application information www.eriks.be www.o-ring.info SEALING ELEMENTS Content Oil & Gas Industry Applications 3 Semiconductor Industry Applications 6 Chemical & Petrochemical Industry Applications 7 Food & Beverage Industry Applications 8 Potable Water Applications 9 Automotive Industry Applications 10 Pharmaceutical, Analytical & Biotechnical Applications 11 Responsibility All information published in this brochure was collected with the utmost care and precision. Despite of this, ERIKS cannot and will not accept any liability claims, originating from possible incompleteness and/or inadequacy of the contents of this brochure. All information included herein is subject to change without prior notice. 2 APPLICATION INFORMATION 1. Oil & Gas Chemical Compatibility Description Solutions Applications in the Oil & Gas Industry Regulating agencies for this industry range from seals and components are primarily the American Petroleum involved in exploration and drilling to Institute (AP) Series 6 - Valves and high-pressure / high-temperature Wellhead Equipment. sealing components in down-hole wells. Extreme temperatures and pressures place great demands on ela- stomers for this industry. In addition, many chemicals either experienced in the reservoir, or used in the drilling, completion or intervention process can degrade many common elastomers. Oil & Gas Industry Applications Segments Environment Elastomers Sealing Considerations Drilling -40 to 250 F HNBR, FKM Abrasion resistance, low compression set, to 7,500 psi drilling mud -

Sealing Solutions for the Chemical Industry Table of Contents
SEALING SOLUTIONS FOR THE CHEMICAL INDUSTRY TABLE OF CONTENTS 1. INTRODUCTION 3 The Company 3 The chemical industry and its requirements 4 2. SCHEMATIC PROCESS PLANT 6 3. OVERVIEW OF MATERIAL AND PRODUCT PORTFOLIO 8 4. TECHNICAL PLASTICS AND THEIR APPLICATIONS 10 PTFE 11 ePTFE 13 POM, PA, PEEK 14 5. ELASTOMERS AND THEIR APPLICATIONS 15 FKM 16 SIMRIZ 17 EPDM 18 6. PRODUCT PORTFOLIO 19 O-rings 20 Flat gaskets 21 Guide rings 22 Chevron seals 23 Packings 24 Valtec 25 Rotary Shaft Seals 26 PTFE bellows 27 U-cup rings 28 PTFE inlet nozzles 29 Molded parts 30 Diaphragms and Profiles 31 2 Introduction INTRODUCTION PLANT PROCESS SCHEMATIC PORTFOLIO MATERIAL PRODUCT AND PLASTICS THE COMPANY APPLICATIONS THEIR TECHNICAL AND Founded in 1849, the Freudenberg Group is a global tech- From a defined standard product range to customer-specific nology company that remains family-owned until today. developments, Freudenberg Sealing Technologies offers a Consisting of more than 30 market segments, it develops unique product portfolio. technically leading products for a wide range of applica- tions in cooperation with their customers – from sealing Freudenberg Sealing Technologies' market sector process technology through nonwovens to chemicals and medical industry offers sealing solutions especially for the areas: devices. • Food & beverage industry • Chemical industry Freudenberg Sealing Technologies is the largest business • Pharmaceutical industry APPLICATIONS group of the Freudenberg Group. Actin as a supplier, devel- oper and service partner. As a globally leading technology These segments are characterized by high demands on ELASTOMERS THEIR specialist, Freudenberg Sealing Technologies serves sectors the sealing portfolio and the applied materials. -

Ball Valve Seat/Seal Materials and Service Applications
APPLICATIONS - VALVE SOFT SEAT/SEAL MATERIALS One of the most important factors affecting shutoff capability is the nature of media being handled. Service life is affected by all of the following factors: - pressure, temperature, degree of pressure fluctuation and thermal fluctuation, type of media, cycling frequency, velocity of media & speed of valve operation. The following seat & seal materials can be used in various valves such as ball, plug, butterfly, needle, etc. BUNA-N (HYCAR or Nitrile) - Buna-N is a general-purpose polymer which has good resistance to oil, water, solvents and hydraulic fluids. It also displays good compression, abrasion resistance, and tensile strength. This material performs extremely well in process areas where paraffin base materials, fatty acids, oils, alcohols or glycerins are present, since it is totally unaffected. It should not be used around high polar solvents (acetones, ketones), chlorinated hydrocarbons, ozone or nitro hydrocarbons. Temperature range is 107°C maximum. Hycar is black in colour and should not be used where discolouration cannot be tolerated. It is regarded as a comparable replacement neoprene. Major differences are: Buna-N has a higher temperature limit; neoprene is more resistant to oils. EPDM - EPDM is a terpolymer elastomer made from ethylene-propylene diene monomer. EPDM has good abrasion and tear resistance and offers excellent chemical resistance to a variety of acids and alkalines. It is susceptible to attacks by oils and is not recommended for applications involving petroleum oils, strong acids, or strong alkalines. EPDM should not be used on compressed air lines. It has exceptionally good weather aging and ozone resistance. -
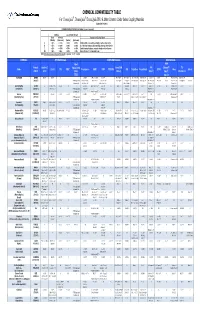
CPC Chemical Compatibility Chart
CHEMICAL COMPATIBILITY TABLE For ChemQuik®, DrumQuik®, DrumQuik PRO & Other Common Colder Series Coupling Materials (Updated 01/14/2010) INTERPRETATION OF TEST DATA (In 30 days to 1 year of exposure) Swelling Loss of Tensile Strength Linear Volumetric Description of Chemical Attack (Plastics) (Elastomers) (Plastics) (Elastomers) A < 10% <= 15% < 15% <=15% Excellent, little or no swelling, softening or surface deterioration B < 15% <= 30% < 30% <= 30% Good chemical resistance, minor swelling, softening or deterioration C < 20% <= 50% < 50% <= 60% Limited chemical resistance, moderate attack, conditional service NR > 20% > 50% > 50% > 60% Severe attack, not recommended for use NOTE: All temperatures are in degrees Fahrenheit. Conversion: °C = (°F - 32)/1.8 CHEMICAL SPRING Materials COUPLING Materials SEAL Materials Teflon® FFKM ® Formula Hastelloy C Encapsulated Acetal/POM FKM (Chemraz / TPO Name 316 SS PPS PEEK™ Polypropylene HDPE PVDF PTFE/PFA ABS Polysulfone Polycarbonate EPDM Buna Silicone (CAS #) (276) 316SS (Celcon) (Viton®) Simriz® / (Santoprene) (TESS) Kalrez®) Acetic Acid C2H4O2 A to 212° 212° A to 212° 212° A A A A to 140° 140° AB to 100% to 70° 70° A to 122° 122° A AAto5%to70 to 5% to 70°° AB 10% to 70° 70° A to 100% to 70° 70° A to 50% to 70° 70° A 10% to 70° 70° AAto70 to 70°° A B to 30% at 70° 70° A to 30% to 70° 70° A (64-19-7) (PTFE Encapsulated AB 50-100% to 160° AB 60% to 180° A to 10% to 225° BC 10% @ 70° C 20% @ 70° A to 20% to 140° B to 50% @ 122° B 10-25% to 100° AB to 200° A to 70° B to 20% to 185° C 50% @ 70° A to