Complex and Curcumin Backboned Polyprodrugs
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
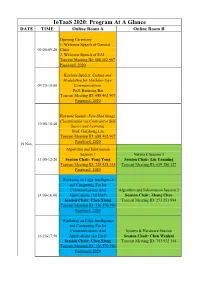
Iotaas 2020: Program at a Glance DATE TIME Online Room a Online Room B
IoTaaS 2020: Program At A Glance DATE TIME Online Room A Online Room B Opening Ceremony 1. Welcome Speech of General 09:00-09:20 Chair 2. Welcome Speech of EAI Tencent Meeting ID: 688 462 967 Password: 2020 Keynote Speech: Coding and Modulation for Machine-Type 09:20-10:00 Communications Prof. Baoming Bai Tencent Meeting ID: 688 462 967 Password: 2020 Keynote Speech: Few-Shot Image Classification via Contrastive Self- 10:00-10:40 Supervised Learning Prof. Guizhong Liu Tencent Meeting ID: 688 462 967 Password: 2020 19 Nov. Algorithm and Information Session 1 Network Session 1 11:00-12:20 Session Chair: Fang Yong Session Chair: Liu Yanming Tencent Meeting ID: 725 528 335 Tencent Meeting ID: 639 386 127 Password: 2020 Workshop on Edge Intelligence and Computing For Iot Communications And Algorithm and Information Session 2 14:00-16:00 Applications (1st Harf) Session Chair: Zhang Chen Session Chair: Chen Xiang Tencent Meeting ID: 273 251 994 Tencent Meeting ID: 156 570 396 Password: 2020 Workshop on Edge Intelligence and Computing For Iot Communications And System & Hardware Session 16:10-17:50 Applications (2st Harf) Session Chair: Chen Wenhui Session Chair: Chen Xiang Tencent Meeting ID: 751 932 354 Tencent Meeting ID: 156 570 396 Password: 2020 Workshop on Satellite Application Session Communications Session Chair: Abdullah Ghaleb 09:00-10:20 and Spatial Information Network Tencent Meeting ID: 672 146 720 Session Chair: Li Jingling Password: 2020 Tencent Meeting ID: 239 301 133 Network Session 2 Artificial Intelligence Session Session Chair: Li Bo Session Chair: Zhang Jingya 10:30-11:50 Tencent Meeting ID: 515 118 859 Tencent Meeting ID: 922 725 202 Password: 2020 Password: 2020 20 Nov. -

Ieee Icet 2020
IEEE ICET 2020 CONFERENCE PROGRAM 2020 IEEE The 3rd International Conference on Electronics Technology Organized by Patrons Sponsored by Media Support 0 About IEEE ICET International Conference on Electronics Technology (IEEE ICET) which is yearly held in Chengdu, China. It is organized by Sichuan Institute of Electronics, sponsored by IEEE, also with the support of University of Electronic Science and Technology of China, Sichuan University, Southwest Jiaotong University and Singapore Institute of Electronics. TABLE OF CONTENTS We sincerely hope that IEEE ICET will provide a WELCOME 2 platform for all delegates to have rich, useful, and effective deliberations that can lead to international CONFERENCE AT A GLANCE 3 cooperation. ORGANIZING COMMITTEE 4 PREPARATION FOR ONLINE 7 CONFERENCE PRESENTATION GUIDELINE 8 TEST SESSIONS AT A GLANCE 9 KEYNOTE AND INVITE SPEECHES AT 11 A GLANCE KEYNOTE SPEECH ABSTRACTS 14 Basic protective measures against INVITED SPEECH ABSTRACTS 20 the COVID-19 from WHO PARALLEL SESSIONS AT A GLANCE 36 Wash your hands frequently Maintain social distancing PARALLEL SESSION DETAILS 39 Avoid touching eyes, nose and mouth Practice respiratory hygiene CO-SPONSORS AND PATRONS ON COVER If you have fever, cough and difficulty breathing, seek medical care early Stay informed and follow advice given by your healthcare provider 1 Welcome It is indeed a pleasure to welcome all participants of the 2020 IEEE 3rd International Conference on Electronics Technology (IEEE ICET). The conference is organized by Sichuan Institute of Electronics, sponsored by IEEE, also with the support of University of Electronic Science and Technology, Sichuan University, Southwest Jiaotong University and Singapore Institute of Electronics. -

Emergency Disaster Preparedness
Fuzhou University: Emergency Disaster Preparedness University of Southern California Sol Price School of Public Policy Policy, Planning, and Development International Laboratory 3 June 2016 Fuzhou, Fujian Province Authors: Natalie Dial, IPPAM ([email protected]) Meredith Fear, MPA ([email protected]) Maggie Ferrill, MPA ([email protected]) Bevin Kloepper, MPA ([email protected]) Steve D. Martinez, IPPAM ([email protected]) Jaime A.Varela, MPA ([email protected]) Supervising Instructor: Dr. Eric Heikkila, PhD Contributing Partners: Fuzhou University, Fujian Province Flood Command and Drought Control Center, Fujian Province Earthquake Administration Center, Fujian Province Public Service Administration Center Acknowledgements: The authors would like to thank the following people for their contributions in translation, data collection, and insight for the final report: Chen Liping (Chuck) Chen Xiaoyue (Joy) Gao Yanxin (Gary) Lai Xinyan (Cherry) Liu Yuxin (Grace) Xie Xiaojuan (Sherry) Yin Xiaoyan (Elli) Table of Contents I. Introduction to Disasters in China and Fuzhou II. Research Orientation III. China, Fuzhou, & Emergency Management A. Governance Context B. Three Dimensional Approach to Utilizing Technology in China C. International Technological Cooperation IV. Traditional and Modern Community Networks V. Emerging Global Technology A. Cutting Edge Apps B. The Cloud C. Projectemergency.lu VI. Vulnerable Populations in China A. Vulnerability Indicators B. Profiles of Vulnerable Populations in China VII. Interviews and Surveys A. Profile Development B. Survey Numbers and Data C. Survey Flaws D. Scaling Up VIII. Fuzhou University & Emergency Management A. Research & Next Steps IX. Conclusion I. Introduction to Disasters in China and Fuzhou The Fujian Province, with its lush and mountainous landscape, is susceptible to earthquakes, floods and subsequent landslides. -

Insight Into Hyper-Branched Aluminum Phosphonate in Combination with Multiple Phosphorus Synergies for Fire-Safe Epoxy Resin Composites
Supplementary Information Insight into Hyper-Branched Aluminum Phosphonate in Combination with Multiple Phosphorus Synergies for Fire-Safe Epoxy Resin Composites Yao Yuan 1, Bin Yu 2, Yongqian Shi 3, Long Mao 1, Jianda Xie 1, Haifeng Pan 4, Yuejun Liu 1,* and Wei Wang 5,* 1 Fujian Provincial Key Laboratory of Functional Materials and Applications, School of Materials Science and Engineering, Xiamen University of Technology, Xiamen 361024, People′s Republic of China; [email protected] (Y.Y.); [email protected] (L.M.); [email protected] (J.X.) 2 Centre for Future Materials, University of Southern Queensland, Toowoomba, QLD 4350, Australia; [email protected] 3 College of Environment and Resources, Fuzhou University, Fuzhou 350002, People′s Republic of China; [email protected] 4 Faculty of Engineering, China University of Geosciences (Wuhan), Wuhan, Hubei, 430074, People′s Republic of China; [email protected] 5 State Key Laboratory of Fire Science, University of Science and Technology of China, Hefei, 230026, People′s Republic of China * Correspondence: [email protected] (W.W.); [email protected] (Y.L.) Characterization 1H and 31P nuclear magnetic resonance (1H and 31P NMR) spectra were recorded on an AVANCE 400 Bruker spectrometer at room temperature using DMSO-d and D2O as the solvent, respectively. Fourier transform infrared (FTIR) spectra were obtained by a Nicolet 6700 spectrometer (Nicolet Instrument Company, USA) using KBr pellets. The wavenumber range was 400–4000 cm−1 and the resolution was 4 cm−1. The crystal-phase properties of the samples were analyzed with a powder X-ray diffractometer (XRD) (Japan Rigaku D Max-Ra) using a rotating anode X-ray diffractometer equipped with a Ni filtered Cu-Ka tube (λ = 1.54178 Å) in the 2θ range from 10° to 70° with a scanning rate of 4 min−1. -

C:\Users\Wind\Documents\Tencent Files\1322870972\Filerecv\IJES
The 8th Asia-Pacific Services Computing Conference APSCC 2014 http://grid.hust.edu.cn/apscc2014 Fujian West Lake Hotel Fuzhou, China December 4-6, 2014 Program Sponsored by Message from Program Chairs Welcome to APSCC 2014, the Eighth Asia-Pacific Services Computing Conference. APSCC 2014 is an important forum for researchers and industry practitioners to exchange information regarding advancements in the state of art and practice of Services Computing, as well as to identify emerging research topics and define the future directions of Services Computing. This year, APSCC attracted 205 submissions. The task of selecting papers involved more than 160 members from 13 countries/regions of the Program Committee. Almost all the papers were peer reviewed by at least three referees. After a thorough examination, 55 papers were accepted in the main track, which results in an acceptance ratio of 26.8%. Beside the main track, APSCC 2014 launched five special tracks, focusing on various emerging topics related to services computing. The papers submitted to each special track were examined by the separated PC committee. After a thorough examination, 34 papers were accepted by the five special tracks. The acceptance ratio of the special tracks is 30.4%. APSCC 2014 has a large number of additional components over the main conference, which greatly adds to its attractiveness. The conference starts with the keynote by Professor Carl K. Chang, 2004 President of IEEE Computer Society, and the winner of CCF Award of Excellent Contribution for Overseas Chinese, and the keynote by Dr. Zhen Liu, Director and Head of China Innovation Group, Microsoft Asia R&D Center. -

17-21 April 2017, Beijing, China Edited by Junwei Cao, Jiye Wang, Wenhua Liu, and Kai Xie
17-21 April 2017, Beijing, China Edited by Junwei Cao, Jiye Wang, Wenhua Liu, and Kai Xie CONFERENCE INFORMATION PAPERS BY SESSION PAPERS BY AUTHOR GETTING STARTED TRADEMARKS SEARCH PROCEEDINGS First IEEE International Conference on Energy Internet — ICEI 2017 — 17–21 April 2017 Beijing, China PROCEEDINGS First IEEE International Conference on Energy Internet — ICEI 2017 — 17–21 April 2017 Beijing, China Edited by Junwei Cao Jiye Wang Wenhua Liu Kai Xie Los Alamitos, California Washington • Tokyo Copyright © 2017 by The Institute of Electrical and Electronics Engineers, Inc. All rights reserved. Copyright and Reprint Permissions: Abstracting is permitted with credit to the source. Libraries may photocopy beyond the limits of US copyright law, for private use of patrons, those articles in this volume that carry a code at the bottom of the first page, provided that the per-copy fee indicated in the code is paid through the Copyright Clearance Center, 222 Rosewood Drive, Danvers, MA 01923. Other copying, reprint, or republication requests should be addressed to: IEEE Copyrights Manager, IEEE Service Center, 445 Hoes Lane, P.O. Box 133, Piscataway, NJ 08855-1331. The papers in this book comprise the proceedings of the meeting mentioned on the cover and title page. They reflect the authors’ opinions and, in the interests of timely dissemination, are published as presented and without change. Their inclusion in this publication does not necessarily constitute endorsement by the editors, the IEEE Computer Society, or the Institute of Electrical and Electronics Engineers, Inc. IEEE Computer Society Order Number: E6110 BMS Part Number: CFP17D74-USB ISBN-13: 978-1-5090-5759-7 Additional copies may be ordered from: IEEE Computer Society IEEE Service Center IEEE Computer Society Customer Service Center 445 Hoes Lane Asia/Pacific Office 10662 Los Vaqueros Circle P.O. -

Journal of Fuel Chemistry and Technology
JOURNAL OF FUEL CHEMISTRY AND TECHNOLOGY AUTHOR INFORMATION PACK TABLE OF CONTENTS XXX . • Description p.1 • Audience p.1 • Abstracting and Indexing p.1 • Editorial Board p.1 • Guide for Authors p.7 ISSN: 1872-5813 DESCRIPTION . The Journal of Fuel Chemistry and Technology publishes reports of both basic and applied research in the chemistry and chemical engineering of many energy sources, including those involved in: The nature, processing and utilization of coal, petroleum, oil, shale, natural gas and biomass Applied catalytic research in the chemistry of synthesis of new or alternative energy sources, such as hydrogen energy, fuel cell, synfuels, biofuels and C1 chemistry Related research includes pollution control and environmental protection during fuel conversion and utilization processing, such as elimination of environmentally hazardous effluents, waste (refuse)-derived fuels and waste treatments Types of publications include original research articles, short communications, research notes and reviews. Manuscripts written in English or Chinese will be accepted. Please submit your manuscripts to http://rlhxxb.sxicc.ac.cn. Benefits to authors We also provide many author benefits, such as free PDFs, a liberal copyright policy, special discounts on Elsevier publications and much more. Please click here for more information on our author services. Please see our Guide for Authors for information on article submission. If you require any further information or help, please visit our Support Center AUDIENCE . The Journal of Fuel Chemistry -

The Relationship Between the Facial Expression of People in University Campus and Host- City Variables
Supplementary: The relationship between the facial expression of people in university campus and Host- city variables Hongxu Wei 1, Richard J. Hauer 2 and Xuquan Zhai 3,* 1 Northeast Institute of Geography and Agroecology, Chinese Academy of Sciences, Changchun 130102, China; [email protected] 2 College of Natural Resources, University of Wisconsin–Stevens Point, 800 Reserve St., Stevens Point, WI 54481, United States; [email protected] 3 China Center for Public Sector Economy Research, Jilin University, Room 3007, Kuang, Yaming Building, 2699 Qianjin Road, Chaoyang District, Changchun 130012, China, Changchun 130012, China; * Correspondence: [email protected]; Tel.: +86-431-8516-8829 Figure S1. The panel of FireFACETM-V1.0 to recognize photos with typically happy, sad, and neutral facial expressions. The model is Ryōko Hirosue from Japan. Photos are downloaded from following websites: Happy: https://images.app.goo.gl/Kh5FgWEMMSPUa3sD9 Sad: https://images.app.goo.gl/GazwjvTh8a6qiyBa9 Neutral: https://images.app.goo.gl/Hn2gtLonVpoLd5o27 Figure S2. The copyright of the FireFACETM-V1.0 software that is authorized in mainland China. Table S1. The list of key universities in the 211-Project of mainland China with Province and City names. Rank Province City University name 1 Anhui University 2 Anhui Hefei Hefei University of Technology 3 University of Science and Technology of China 4 Beijing Foreign Studies University 5 Beijing Forestry University 6 Beijing Institute of Technology 7 Beijing Jiaotong University 8 Beijing Normal University -

Initiative Disciplines Development List 01 Natural and Physical Sciences (100)
“Double First-Class” initiative disciplines development list (Sorted by discipline) Note: The list was published by the Ministry of Education of the People’s Republic of China on 21 September 2017. This list sorted by discipline has been prepared by the Education and Research Section, Australian Embassy, Beijing based on the Australian Standard Classification of Education (ASCED) 2001, Australian Bureau of Statistics. Initial translation credit to Science, Technology and Education Section, Embassy of Switzerland in China. 01 Natural and Physical Sciences (100) Astronomy (2) Nanjing University University of Science and Technology of China Atmosphere Science (3) Nanjing University Nanjing University of Information Science & Technology Lanzhou University Biology (16) Peking University Tsinghua University China Agricultural University Peking Union Medical College Inner Mongolia University Fudan University Shanghai Jiao Tong University Nanjing University Zhejiang University University of Science and Technology of China Xiamen University Huazhong Agricultural University Henan University Sun Yat-sen University Wuhan University Southwest University Chemistry (25) Peking University Tsinghua University Nankai University Tianjin University Dalian University of Technology Jilin University Northeast Normal University Fudan University Shanghai Jiao Tong University East China University of Science and Technology Nanjing University Zhejiang University Xiamen University University of Science and Technology of China Fuzhou University Shandong University -

Program 11-13 October 2019·Nanjing·China
The 12th International Workshop on Complex-sys- tems for Future Technologies and Applications (IWCFTA 2019) Program 11-13 October 2019·Nanjing·China Sponsors Jiangsu Provincial Key Laboratory of Networked Collective Intelligence, Southeast University Technical Committee on Network Science and Engineering of Chinese Institute of Command and Control City University of Hong Kong Technical support: IEEE CASS (Nonlinear CAS Technical Committee) Northeastern University Conference site: Nanjing Zhongshan Hotel Website: https://nci.seu.edu.cn/wome_23639/list.htm Index General Information .......................................................................................................................................... 5 Organizing Committee ..................................................................................................................................... 6 Honorary Chairs ......................................................................................................................................... 6 General Chairs ............................................................................................................................................ 6 General Co-Chairs .................................................................................................................................... 6 Program Chairs .......................................................................................................................................... 6 Program Co-Chairs ................................................................................................................................. -

Handbook for Foreign Students
Handbook for Foreign Students Overseas Education College of Fuzhou University Fuzhou·China Catalog Message from the President ··························1 Location of FZU ··························2 Map of New Campus ··························5 Introduction of Fuzhou ·························7 Introduction of Fuzhou University ····················10 Rules of Conduct ························12 Campus Life ························13 Scholarships ·························13 Tuitions and Fees ························14 A Glimpse of the Dormitory ························15 Dormitory notice ························16 Enrollment Requirements and Application Procedures ··17 Special Safety Notice ·····················18 Residence Permit Application for Freshmen ···········19 Q & A ························21 Message from the President Dear Students, First, I would like to express my warm welcome to all of you! Fuzhou University (FZU) is located at the foot of Qishan Mountain beside Wulong River. With beautiful scenery and fresh air, you may feel she has only spring in one year. Moreover, towering mountains and gurgling rivers reveal a beautiful and glorious history. FZU has gathered the respect of teachers, national academicians, scholars, as well as national and provincial famous tutors. Lectures, debates and discussions create a stimulating learning environment. Our world-class resources will be your most valuable assets towards growth and success. FZU places great importance on international campus construction. The colorful and diverse campus cultural activities will provide you a fulfilling extracurricular life. Welcome to FZU. Wish you a great success here! 1 Location of FZU By Airplane It is one-hour-and-a-half drive from the university to Fuzhou Changle International Airport. There are direct flights from Fuzhou to main cities in China as well as international flights to Hong Kong, and other cities. You can get to Changle Airport by taxi,or by bus. -

“Disaster Resilience in Fuzhou, China” USC Price China Lab PPD 613A and 613B Spring and Summer 2016
USC Sol Price School of Public Policy “Disaster Resilience in Fuzhou, China” USC Price China Lab PPD 613a and 613b Spring and Summer 2016 Instructor Eric Heikkila, Professor & Spring meeting dates: Director, International Initiatives Jan 21, Feb 18, March 24, April 14 USC Price School of Public Policy 6pm – 9pm; RGL 103 University of Southern California Dates in Fuzhou: Office Hours: TBD May 23 – June 3 Email: [email protected] USC Price International Labs The Price School International Labs integrate scholarly knowleDge with professional practice by providing consulting services in a setting outside the United States. Lab participants work collaboratively in multidisciplinary teams to aDDress a particular project identifieD by the client in the host country. With active guiDance from their professor, stuDents analyze information pertinent to the project anD its context, anD then proDuce a set of policy recommenDations for the client. These recommenDations are supporteD by classroom knowleDge, academic research, analytical tools, databases, case stuDies, together with various maps anD graphics. As participants transition from the classroom to a real-worlD international setting, they gain Direct experience with translating professional practice in a cross-cultural context. While some backgrounD research anD preparation is necessary prior to leaving the U.S., the bulk of the assignment is unDertaken on an intensive basis in the field. The on-site work culminates in a presentation to the client. The International Labs are designeD as integrative professional experiences for graDuate stuDents from across the Price School. GraDuate stuDents from other USC programs may also join on a case-by-case basis (with permission from the instructor).