FDA Approval Letter
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Top Twenty Pay-For-Delay Drugs: How Drug Industry Payoffs Delay Generics, Inflate Prices and Hurt Consumers
TOP TWENTY PAY-FOR-DELAY DRUGS: HOW DRUG INDUSTRY PAYOFFS DELAY GENERICS, INFLATE PRICES AND HURT CONSUMERS oo often, consumers are forced to shoulder a heavy financial burden, or even go without needed medicine, due to the high cost of brand-name drugs. Our research indicates that one significant cause is Tthe practice called “pay for delay,” which inflates the drug prices paid by tens of millions of Americans. In a pay-for-delay deal, a brand-name drug company pays off a would-be competitor to delay it from selling a generic version of the drug. Without any competition, the brand-name company can continue demanding high prices for its drug. This list of 20 drugs known to be impacted by pay-for-delay deals represents the tip of the iceberg. Annual reports by the Federal Trade Commission (FTC) indicate that generic versions of as many as 142 brand-name drugs have been delayed by pay-for-delay arrangements between drug manufacturers since 2005.1 However, because the details of these deals rarely become public, consumers have largely been kept in the dark about the extent of the problem. Information about these twenty specific drugs affected by pay-for-delay deals has been made public thanks to legal challenges brought by the FTC, consumer class action lawsuits, research by legal experts, and public disclosures by drug makers. Key findings of our analysis of these 20 drugs impacted by pay-for-delay deals: . This practice has held back generic . These brand-name drugs cost medicines used by patients with a wide 10 times more than their generic range of serious or chronic conditions, equivalents, on average, and as much as ranging from cancer and heart disease, to 33 times more. -

ORTHO TRI-CYCLEN® TABLETS ORTHO-CYCLEN® TABLETS (Norgestimate/Ethinyl Estradiol)
PHYSICIANS' PACKAGE INSERT ORTHO TRI-CYCLEN® TABLETS ORTHO-CYCLEN® TABLETS (norgestimate/ethinyl estradiol) Patients should be counseled that this product does not protect against HIV infection (AIDS) and other sexually transmitted diseases. DESCRIPTION Each of the following products is a combination oral contraceptive containing the progestational compound norgestimate and the estrogenic compound ethinyl estradiol. ORTHO TRI-CYCLEN 21 Tablets and ORTHO TRI-CYCLEN 28 Tablets. Each white tablet contains 0.180 mg of the progestational compound, norgestimate (18,19-Dinor- 17-pregn-4-en-20-yn-3-one,17-(acetyloxy)-13-ethyl-, oxime,(17α)-(+)-) and 0.035 mg of the estrogenic compound, ethinyl estradiol (19-nor-17α-pregna,1,3,5(10)-trien-20-yne-3,17-diol). Inactive ingredients include lactose, magnesium stearate, and pregelatinized starch. Each light blue tablet contains 0.215 mg of the progestational compound norgestimate (18,19- Dinor-17-pregn-4-en-20-yn-3-one,17-(acetyloxy)-13-ethyl-,oxime,(17α)-(+)-) and 0.035 mg of the estrogenic compound, ethinyl estradiol (19-nor-17α-pregna,1,3,5(10)-trien-20-yne-3,17-diol). Inactive ingredients include FD & C Blue No. 2 Aluminum Lake, lactose, magnesium stearate, and pregelatinized starch. Each blue tablet contains 0.250 mg of the progestational compound norgestimate (18,19-Dinor-17- pregn-4-en-20-yn-3-one, 17-(acetyloxy)-13-ethyl-,oxime,(17α)-(+)-) and 0.035 mg of the estrogenic compound, ethinyl estradiol (19-nor-17α-pregna,1,3,5(10)-trien-20-yne-3,17-diol). Inactive ingredients include FD & C Blue No. 2 Aluminum Lake, lactose, magnesium stearate, and pregelatinized starch. -

Estrogen and Progestin Hormone Doses in Combined Birth Control Pills
Estrogen and Progestin Hormone Doses in Combined Birth Control Pills Estrogen level Pill Brand Name Progestin Dose (mg) ethinyl estradiol (micrograms) 20 mcgm Alesse® levonorgestrel 0.10 Levlite® levonorgestrel 0.10 Loestrin 1/20® Fe norethindrone 1.00 acetate Mircette® desogestrel 0.15 Ortho Evra® norelgestromin 0.15 (patch) (norgestimate metabolite) phasic Estrostep® Fe norethindrone 1.0/1.0/1.0 20/30/35 mcgm acetate 30 mcgm Levlen® levonorgestrel 0.15 Levora® levonorgestrel 0.15 Nordette® levonorgestrel 0.15 Lo/Ovral® norgestrel 0.30 Desogen® desogestrel 0.15 Ortho-Cept® desogestrel 0.15 Loestrin® 1.5/30 norethindrone 1.50 acetate Yasmin® drospirenone 3.0 phasic Triphasil® levonorgestrel 0.05/0.075/0.125 30/40/30 mcgm Tri-Levlen® levonorgestrel 0.05/0.075/0.125 Trivora® levonorgestrel 0.05/0.075/0.125 35 mcgm Ortho-Cyclen® norgestimate 0.25 Ovcon-35® norethindrone 0.40 Brevicon® norethindrone 0.50 Modicon® norethindrone 0.50 Necon® norethindrone 1.00 Norethin® norethindrone 1.00 Norinyl® 1/35 norethindrone 1.00 Ortho-Novum® 1/35 norethindrone 1.00 Demulen® 1/35 ethynodiol diacetate 1.00 Zovia® 1/35E ethynodiol diacetate 1.00 phasic Ortho-Novum® norethindrone 0.50/1.00 35/35 mcgm 10/11 Jenest® norethindrone 0.50/1.00 phasic Ortho-Tri-Cyclen® norgestimate 0.15/0.215/0.25 35/35/35 mcgm Ortho-Novum® norethindrone 0.50/0.75/1.00 7/7/7 Tri-Norinyl® norethindrone 0.50/1.00/0.50 50 mcgm Necon® 1/50 norethindrone 1.00 Norinyl® 1/50 norethindrone 1.00 Ortho-Novum® 1/50 norethindrone 1.00 Ovcon-50® norethindrone 1.00 Ovral® norgestrel 0.50 Demulen® 1/50 ethynodiol diacetate 1.00 Zovia® 1/50E ethynodiol diacetate 1.00 Which pills have higher progestin side efects or cause more acne and hair growth? Each progestin has a diferent potency, milligram per milligram, in terms of progesterone efect to stop menstrual bleeding or androgen efect to stimulate acne and hair growth. -

Merck & Co., Inc
As filed with the Securities and Exchange Commission on February 25, 2021 UNITED STATES SECURITIES AND EXCHANGE COMMISSION WASHINGTON, D. C. 20549 _________________________________ FORM 10-K (MARK ONE) ☒ Annual Report Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 For the Fiscal Year Ended December 31, 2020 OR ☐ Transition Report Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 For the transition period from to Commission File No. 1-6571 _________________________________ Merck & Co., Inc. 2000 Galloping Hill Road Kenilworth New Jersey 07033 (908) 740-4000 New Jersey 22-1918501 (State or other jurisdiction of incorporation) (I.R.S Employer Identification No.) Securities Registered pursuant to Section 12(b) of the Act: Title of Each Class Trading Symbol(s) Name of Each Exchange on which Registered Common Stock ($0.50 par value) MRK New York Stock Exchange 1.125% Notes due 2021 MRK/21 New York Stock Exchange 0.500% Notes due 2024 MRK 24 New York Stock Exchange 1.875% Notes due 2026 MRK/26 New York Stock Exchange 2.500% Notes due 2034 MRK/34 New York Stock Exchange 1.375% Notes due 2036 MRK 36A New York Stock Exchange Number of shares of Common Stock ($0.50 par value) outstanding as of January 31, 2021: 2,530,315,668. Aggregate market value of Common Stock ($0.50 par value) held by non-affiliates on June 30, 2020 based on closing price on June 30, 2020: $195,461,000,000. Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. -
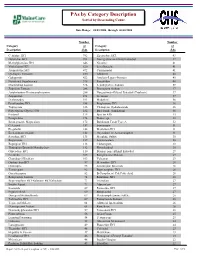
Report 752 by Category Description
PAs by Category Description Sorted by Descending Count Date Range: 04/01/2006 through 06/30/2006 Number Number Category of Category of Description PAs Description PAs Cetirizine HCl 792 Ziprasidone HCl 43 Duloxetine HCl 784 Norelgestromin-Ethinyl Estradiol 42 Methylphenidate HCl 646 Nicotine 41 Venlafaxine HCl 620 Levofloxacin 41 Atomoxetine HCl 472 Carisoprodol 41 Quetiapine Fumarate 430 Albuterol 40 Gabapentin 422 Amylase-Lipase-Protease 40 Nutritional Supplements 378 Famotidine 40 Montelukast Sodium 326 Levothyroxine Sodium 39 Zolpidem Tartrate 288 Enoxaparin Sodium 37 Amphetamine-Dextroamphetamine 286 Norgestimate-Ethinyl Estradiol (Triphasic) 37 Aripiprazole 271 Tretinoin 37 Desloratadine 191 Modafinil 36 Fexofenadine HCl 188 Pioglitazone HCl 36 Topiramate 186 Citalopram Hydrobromide 36 Polyethylene Glycol 3350 182 Budesonide (Inhalation) 36 Fentanyl 175 Epoetin Alfa 33 Eszopiclone 174 Etanercept 33 Esomeprazole Magnesium 172 Botulinum Toxin Type A 32 Celecoxib 157 Somatropin 31 Pregabalin 148 Metformin HCl 31 Escitalopram Oxalate 142 Oxycodone w/ Acetaminophen 31 Sertraline HCl 135 Morphine Sulfate 30 Risperidone 127 Levetiracetam 30 Bupropion HCl 118 Clonazepam 30 Tiotropium Bromide Monohydrate 112 Phenobarbital 30 Oxycodone HCl 110 Drospirenone-Ethinyl Estradiol 29 Ezetimibe 105 Rosiglitazone Maleate 29 Clopidogrel Bisulfate 103 Valsartan 29 Ondansetron HCl 97 Memantine HCl 28 Olanzapine 93 Sumatriptan Succinate 28 Temazepam 92 Buprenorphine HCl 28 Oxcarbazepine 82 B-Complex w/ C & Folic Acid 28 Rabeprazole Sodium 74 Ranitidine -

Cigna Standard 4-Tier Prescription Drug List
CIGNA STANDARD 4-TIER PRESCRIPTION DRUG LIST Coverage as of January 1, 2022 Offered by: Cigna Health and Life Insurance Company, Connecticut General Life Insurance Company, or their affiliates. 916152 j Standard 4-Tier O/I SRx 08/21 What’s inside? About this drug list 3 How to read this drug list 3 How to find your medication 5 Specialty medications 18 Medications that aren’t covered 25 Frequently Asked Questions (FAQs) 41 Exclusions and limitations for coverage 45 View the drug list online This document was last updated on 08/01/2021.* You can go online to see the current list of medications your plan covers. myCigna® App and myCigna.com. Click on the “Find Care & Costs” tab and select “Price a Medication.” Then type in your medication name to see how it’s covered. Cigna.com/PDL. Scroll down until you see a pdf of the Cigna Standard 4-Tier Prescription Drug List (all specialty medications covered on Tier 4). Questions? › myCigna.com: Click to chat Monday-Friday, 9:00 am-8:00 pm EST. › By phone: Call the toll-free number on your Cigna ID card. We’re here 24/7/365. * Drug list created: originally created 01/01/2004 Last updated: 08/01/2021, for changes Next planned update: 03/01/2022, for starting 01/01/2022 changes starting 07/01/2022 2 About this drug list This is a list of the most commonly prescribed medications covered on the Cigna Standard 4-Tier Prescription Drug List as of January 1, 2022.1,2 Medications are listed by the condition they treat, then listed alphabetically within tiers (or cost-share levels). -

Oral Contraceptives
ORAL CONTRACEPTIVES STRENGTH DRUG ESTROGEN PROGESTIN (ESTROGEN/PROGESTIN) MONOPHASIC Aviane 28, Lessina, Lutera, Orsythia, Ethinyl estradiol Levonorgestrel 20mcg/0.1mg Sronyx† Beyaz*, YAZ Ethinyl estradiol Drospirenone 20mcg/3mg [Gianvi, Loryna, Vestura]† Brevicon, Modicon Ethinyl estradiol Norethindrone 35mcg/0.5mg [Necon 0.5/35, Nortrel 0.5/35]† Desogen Ethinyl estradiol Desogestrel 30mcg/0.15mg [Apri, Emoquette, Reclipsen, Solia]† Femcon Fe, Ovcon 35 Ethinyl estradiol Norethindrone 35mcg/0.4mg [Balziva, Briellyn, Philith, Vyfemla Zenchent, Zenchent Fe]† Loestrin 21 1/20, Loestrin Fe 1/20, Ethinyl estradiol Norethindrone acetate 20mcg/1mg Loestrin 24 Fe, Minastrin 24 Fe [Gildess Fe 1/20, Junel 1/20, Junel Fe 1/20, Larin 1/20, Larin Fe 1/20, Microgestin 1/20, Microgestin Fe 1/20]† Loestrin 21 1.5/30, Loestrin Fe 1.5/30 Ethinyl estradiol Norethindrone acetate 30mcg/1.5mg [Gildess Fe 1.5/30, Junel 1.5/30, Junel Fe 1.5/30, Microgestin 1.5/30, Microgestin Fe 1.5/30]† Lo/Ovral Ethinyl estradiol Norgestrel 30mcg/0.3mg [Cryselle, Low-Ogestrel]† Lybrel Ethinyl estradiol Levonorgestrel 20mcg/0.09mg [Amethyst]† [Altavera, Levora, Marlissa, Portia]† Ethinyl estradiol Levonorgestrel 30mcg/0.15mg Norinyl 1/35, Ortho-Novum 1/35 Ethinyl estradiol Norethindrone 35mcg/1mg [Alyacen 1/35, Cyclafem 1/35, Dasetta 1/35, Necon 1/35, Nortrel 1/35]† Necon 1/50, Norinyl 1/50 Mestranol Norethindrone 50mcg/1mg Ogestrel 0.5/50† Ethinyl estradiol Norgestrel 50mcg/0.5mg Ortho-Cyclen Ethinyl estradiol Norgestimate 35mcg/0.25mg [MonoNessa, Previfem, Sprintec]† Ovcon -

Why Are Some Generic Drugs Skyrocketing in Price? Hearing Committee on Health, Education, Labor, and Pensions United States Sena
S. HRG. 113–859 WHY ARE SOME GENERIC DRUGS SKYROCKETING IN PRICE? HEARING BEFORE THE SUBCOMMITTEE ON PRIMARY HEALTH AND AGING OF THE COMMITTEE ON HEALTH, EDUCATION, LABOR, AND PENSIONS UNITED STATES SENATE ONE HUNDRED THIRTEENTH CONGRESS SECOND SESSION ON EXAMINING THE PRICING OF GENERIC DRUGS NOVEMBER 20, 2014 Printed for the use of the Committee on Health, Education, Labor, and Pensions ( Available via the World Wide Web: http://www.gpo.gov/fdsys/ U.S. GOVERNMENT PUBLISHING OFFICE 24–459 PDF WASHINGTON : 2017 For sale by the Superintendent of Documents, U.S. Government Publishing Office Internet: bookstore.gpo.gov Phone: toll free (866) 512–1800; DC area (202) 512–1800 Fax: (202) 512–2104 Mail: Stop IDCC, Washington, DC 20402–0001 VerDate Nov 24 2008 13:32 May 19, 2017 Jkt 000000 PO 00000 Frm 00001 Fmt 5011 Sfmt 5011 S:\DOCS\24459.TXT DENISE HELPN-003 with DISTILLER COMMITTEE ON HEALTH, EDUCATION, LABOR, AND PENSIONS TOM HARKIN, Iowa, Chairman BARBARA A. MIKULSKI, Maryland LAMAR ALEXANDER, Tennessee PATTY MURRAY, Washington MICHAEL B. ENZI, Wyoming BERNARD SANDERS (I), Vermont RICHARD BURR, North Carolina ROBERT P. CASEY, JR., Pennsylvania JOHNNY ISAKSON, Georgia KAY R. HAGAN, North Carolina RAND PAUL, Kentucky AL FRANKEN, Minnesota ORRIN G. HATCH, Utah MICHAEL F. BENNET, Colorado PAT ROBERTS, Kansas SHELDON WHITEHOUSE, Rhode Island LISA MURKOWSKI, Alaska TAMMY BALDWIN, Wisconsin MARK KIRK, Illinois CHRISTOPHER S. MURPHY, Connecticut TIM SCOTT, South Carolina ELIZABETH WARREN, Massachusetts DEREK MILLER, Staff Director LAUREN MCFERRAN, Deputy Staff Director and Chief Counsel DAVID P. CLEARY, Republican Staff Director SUBCOMMITTEE ON PRIMARY HEALTH AND AGING BERNARD SANDERS, Vermont, Chairman BARBARA A. -

Biologic, Biosimilar, Generic, and Brand
BIOSIMILARS Breaking Down the Differences Between Drug Types: Biologic, Biosimilar, Generic, and Brand-Name Drugs BIOSIMILARS This resource is designed to help you understand the differences between biosimilar, biologic, generic, and brand-name drugs so that you can be as informed as possible when it comes to treatment decisions. TABLE OF CONTENTS 2 · Introduction 3 · An Analogy: Cookies & Beer ABOUT FIGHT 4 · Background: Small and COLORECTAL CANCER Large Molecule Drugs We FIGHT to cure colorectal cancer and serve as relentless champions 7 · The Comparisons: of hope for all affected by this Brand-Name vs Generic disease through informed patient & Biologic vs Biosimilar support, impactful policy change, and breakthrough research endeavors. 13 · Making Your Decision 14 · More Information MEDICAL DISCLAIMER & Support The information and services provided by Fight Colorectal Cancer are for general informational purposes only and are not intended to be substitutes for professional medical advice, diagnoses, or treatment. If you are ill, or suspect that you are ill, see a doctor immediately. In an emergency, call 911 or go to the nearest emergency room. Fight Colorectal Cancer never recommends or endorses any specific physicians, products, or treatments for any condition. This mini magazine does not serve as an advertisement or endorsement for any products COVER: or sponsors mentioned. Patrick Moote Stage III survivor 1 • FightCRC.org • INTRODUCTION HILE AT THE PHARMACY OR IN YOUR DOCTOR’S OFFICE, you may have heard the terms “generic” and “brand-name.” WGeneric and brand-name drugs will remain staples in our healthcare system, and there’s a new kid on the block that you may soon encounter: biosimilars. -

Ethinyl Estradiol and Norgestimate
PATIENT & CAREGIVER EDUCATION Ethinyl Estradiol and Norgestimate This information from Lexicomp® explains what you need to know about this medication, including what it’s used for, how to take it, its side effects, and when to call your healthcare provider. Brand Names: US Estarylla; Femynor; Mili; Mono-Linyah; MonoNessa [DSC]; Nymyo; Ortho Tri- Cyclen (28) [DSC]; Ortho Tri-Cyclen Lo [DSC]; Ortho-Cyclen (28) [DSC]; Previfem; Sprintec 28; Tri Femynor; Tri-Estarylla; Tri-Linyah; Tri-Lo-Estarylla; Tri-Lo- Marzia; Tri-Lo-Mili; Tri-Lo-Sprintec; Tri-Mili; Tri-Nymyo; Tri-Previfem; Tri- Sprintec; Tri-VyLibra; Tri-VyLibra Lo; TriNessa (28) [DSC]; TriNessa Lo [DSC]; VyLibra Brand Names: Canada Cyclen [DSC]; Tri-Cira 21; Tri-Cira 28; Tri-Cira Lo 21; Tri-Cira Lo 28; Tri-Cyclen Lo [DSC]; Tri-Cyclen [DSC]; TRI-Jordyna 21; TRI-Jordyna 28 Warning Smoking cigarettes while using this drug raises the chance of severe heart and blood-related side effects. This chance is raised with age (mainly older than 35 years of age). It is also raised with the number of cigarettes smoked. It is strongly advised not to smoke. What is this drug used for? It is used to prevent pregnancy. It is used to treat pimples (acne). It may be given to your child for other reasons. Talk with the doctor. Ethinyl Estradiol and Norgestimate 1/8 What do I need to tell the doctor BEFORE my child takes this drug? If your child is allergic to this drug; any part of this drug; or any other drugs, foods, or substances. Tell the doctor about the allergy and what signs your child had. -

EUROPEAN PHARMACOPOEIA 10.0 Index 1. General Notices
EUROPEAN PHARMACOPOEIA 10.0 Index 1. General notices......................................................................... 3 2.2.66. Detection and measurement of radioactivity........... 119 2.1. Apparatus ............................................................................. 15 2.2.7. Optical rotation................................................................ 26 2.1.1. Droppers ........................................................................... 15 2.2.8. Viscosity ............................................................................ 27 2.1.2. Comparative table of porosity of sintered-glass filters.. 15 2.2.9. Capillary viscometer method ......................................... 27 2.1.3. Ultraviolet ray lamps for analytical purposes............... 15 2.3. Identification...................................................................... 129 2.1.4. Sieves ................................................................................. 16 2.3.1. Identification reactions of ions and functional 2.1.5. Tubes for comparative tests ............................................ 17 groups ...................................................................................... 129 2.1.6. Gas detector tubes............................................................ 17 2.3.2. Identification of fatty oils by thin-layer 2.2. Physical and physico-chemical methods.......................... 21 chromatography...................................................................... 132 2.2.1. Clarity and degree of opalescence of -

Dr Reddy's Prescription to Stay Ahead of the Generic Pack
Dr Reddy’s prescription to stay ahead of the generic pack The company has differentiated itself by focusing on internal processes and staying away from costly acquisitions. Now, it has other plans to fend off the competition B DASARATH REDDY Hyderabad, 8 August BATTLE OF THE GENERICS As the patent cliff in the US levels off, generic drug makers will face increasing pressure to develop other kinds of products he revenues of Hyderabad- Revenues in 2011-12 (Actual) Revenue for top-three Indian based generic drug maker, Pharma companies T Dr Reddy's Laboratories, is expected to surpass those of ~ crore Revenue Net profit ~ crore 2012-13 2013-14* Ranbaxy Laboratories, the largest Ranbaxy 9,977 Ranbaxy 11,309 Indian generic drug company, and Laboratories* -2,900 Laboratories 12,615 now a subsidiary of Japan’s Daiichi Dr Reddy's 9,674 Dr Reddy's 11,286 Sankyo, sometime next year. Laboratories** 1,426 Laboratories 12,663 Barclays Equity Research projects 8,006 9,694 Dr Reddy’s revenues for FY14 at Sun Pharmaceutical Sun Pharmaceutical 2,587 11,472 ~12,663 crore, slightly higher than Industries** Industries Ranbaxy’s (at ~12,615 crore). *Calendar year 2011, **FY ended March 2012 *Estimates This would be big news for any company battling it out in a chal- ANDA* pipeline of top-three companies Global pharmacy lenging industry. However, the Approved Pending Share of consolidated revenues for Dr Reddy's in the year ‘11-12 generic-drug business is more than just that. It’s a fiercely com- 241 petitive business, driven by vol- umes and characterised by con- 148 148 stantly falling prices and, 101 therefore, steep margins.