Androgens in Women: Hormone-Modulating Therapies For
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Compositions Comprising Drospirenone Molecularly
(19) & (11) EP 1 748 756 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: A61K 9/00 (2006.01) A61K 9/107 (2006.01) 29.04.2009 Bulletin 2009/18 A61K 9/48 (2006.01) A61K 9/16 (2006.01) A61K 9/20 (2006.01) A61K 9/14 (2006.01) (2006.01) (2006.01) (21) Application number: 05708751.2 A61K 9/70 A61K 31/565 (22) Date of filing: 10.03.2005 (86) International application number: PCT/IB2005/000665 (87) International publication number: WO 2005/087194 (22.09.2005 Gazette 2005/38) (54) COMPOSITIONS COMPRISING DROSPIRENONE MOLECULARLY DISPERSED ZUSAMMENSETZUNGEN AUS DROSPIRENON IN MOLEKULARER DISPERSION DES COMPOSITIONS CONTENANT DROSPIRENONE A DISPERSION MOLECULAIRE (84) Designated Contracting States: (72) Inventors: AT BE BG CH CY CZ DE DK EE ES FI FR GB GR • FUNKE, Adrian HU IE IS IT LI LT LU MC NL PL PT RO SE SI SK TR D-14055 Berlin (DE) Designated Extension States: • WAGNER, Torsten AL BA HR MK YU 13127 Berlin (DE) (30) Priority: 10.03.2004 EP 04075713 (74) Representative: Wagner, Kim 10.03.2004 US 551355 P Plougmann & Vingtoft a/s Sundkrogsgade 9 (43) Date of publication of application: P.O. Box 831 07.02.2007 Bulletin 2007/06 2100 Copenhagen Ø (DK) (60) Divisional application: (56) References cited: 08011577.7 / 1 980 242 EP-A- 1 260 225 EP-A- 1 380 301 WO-A-01/52857 WO-A-20/04022065 (73) Proprietor: Bayer Schering Pharma WO-A-20/04041289 US-A- 5 569 652 Aktiengesellschaft US-A- 5 656 622 US-A- 5 789 442 13353 Berlin (DE) Note: Within nine months of the publication of the mention of the grant of the European patent in the European Patent Bulletin, any person may give notice to the European Patent Office of opposition to that patent, in accordance with the Implementing Regulations. -

Drospirenone-Containing Birth Control Pills (Yaz, Yasmin, Ocella, Gianvi and Their Generics)
Drospirenone-Containing Birth Control Pills (Yaz, Yasmin, Ocella, Gianvi and their generics) Drospirenone is a progestin component of some birth control pills that does some blocking of testosterone (the "male hormone" that women have, too, and the hormone that can be a part of making acne worse). This is why drospirenone-containing birth control pills are especially good at helping acne (and why they can theoretically help hirsutism and female-pattern scalp hair loss as well). Drospirenone is also a mild diuretic and is why these birth control pills do not cause the water weight gain that some birth control pills can. Any birth control pill can theoretically help acne just by evening out hormonal fluctuations, but for many birth control pills, the effects on acne is very minimal. Which birth control pills are most likely to help acne? Some birth control pills are more like testosterone and some are less like testosterone. The ones that are less like testosterone can theoretically help acne more. The drospirenone-containing birth control pills go one step further by blocking testosterone, and may show the most benefit on clearing acne. Drospirenone is a progestin that is derived from 17-alpha-spironolactone and is chemically related to the diuretic spironolactone. Spironolactone is a diuretic (used for high blood pressure) that is sometimes used off-label for the treatment of acne, hirsutism or female-pattern hair loss. Often people say that a drospirenone-containing birth control pill may be equivalent to 25mg of spironolactone. Risks : Any estrogen-containing birth control pill increases the risk of a high blood pressure, stroke, or other life-threatening blood clot, and one study showed possibly a slightly higher risk with a drospirenone-containing birth control pill. -

Nonsurgical Hair Restoration Treatment
COSMETIC DERMATOLOGY Nonsurgical Hair Restoration Treatment Roya S. Nazarian, BA; Aaron S. Farberg, MD; Peter W. Hashim, MD, MHS; Gary Goldenberg, MD androgenic alopecia (AGA).3 Currently, minoxidil and PRACTICE POINTS finasteride are the only US Food and Drug Administration • Hair loss is a common phenomenon in both men (FDA)–approved medications for the treatment of hair and women and can seriously impact psychosocial loss; however, other nonsurgical treatment options have functioning. gained popularity, including dutasteride, spironolactone, • There are numerous US Food and Drug Administration– low-level laser therapy (LLLT), platelet-rich plasma (PRP), approved and off-label nonsurgical treatment options microneedling, stemcopy cells, and nutraceutical supplements. for alopecia. Dermatologists should be well versed in We provide an overview of these treatment options to these treatment modalities and the associated side- help dermatologists select appropriate therapies for the effect profiles to select the appropriate therapy for treatment of alopecia (Table). each patient. Minoxidilnot Minoxidil has been known to improve hair growth for more than 40 years. Oral minoxidil was first introduced Patterned hair loss is common and can negatively impact quality of for hypertension in the 1970s with a common adverse life. Patients often seek nonsurgical treatment options as a first-lineDo measure to avoid undue risks and expense associated with surgery. effect of hypertrichosis; the 2% solution was marketed for 4 This article discusses these noninvasive treatment options, with a AGA shortly thereafter in 1986. Minoxidil is a biologic focus on minoxidil, finasteride, dutasteride, spironolactone, low-level response modifier that is thought to promote hair growth laser therapy (LLLT), platelet-rich plasma (PRP), microneedling, and through vasodilation and stimulation of hair follicles into oral supplements. -

Download PDF File
Ginekologia Polska 2019, vol. 90, no. 9, 520–526 Copyright © 2019 Via Medica ORIGINAL PAPER / GYNECologY ISSN 0017–0011 DOI: 10.5603/GP.2019.0091 Anti-androgenic therapy in young patients and its impact on intensity of hirsutism, acne, menstrual pain intensity and sexuality — a preliminary study Anna Fuchs, Aleksandra Matonog, Paulina Sieradzka, Joanna Pilarska, Aleksandra Hauzer, Iwona Czech, Agnieszka Drosdzol-Cop Department of Pregnancy Pathology, Department of Woman’s Health, School of Health Sciences in Katowice, Medical University of Silesia, Katowice, Poland ABSTRACT Objectives: Using anti-androgenic contraception is one of the methods of birth control. It also has a significant, non-con- traceptive impact on women’s body. These drugs can be used in various endocrinological disorders, because of their ability to reduce the level of male hormones. The aim of our study is to establish a correlation between taking different types of anti-androgenic drugs and intensity of hirsutism, acne, menstrual pain intensity and sexuality . Material and methods: 570 women in childbearing age that had been using oral contraception for at least three months took part in our research. We examined women and asked them about quality of life, health, direct causes and effects of that treatment, intensity of acne and menstrual pain before and after. Our research group has been divided according to the type of gestagen contained in the contraceptive pill: dienogest, cyproterone, chlormadynone and drospirenone. Ad- ditionally, the control group consisted of women taking oral contraceptives without antiandrogenic component. Results: The mean age of the studied group was 23 years ± 3.23. 225 of 570 women complained of hirsutism. -

Health Risks Associated with Long-Term Finasteride and Dutasteride Use: It’S Time to Sound the Alarm
Review Article Health promotion, disease prevention, and lifestyle pISSN: 2287-4208 / eISSN: 2287-4690 World J Mens Health 2020 Jul 38(3): 323-337 https://doi.org/10.5534/wjmh.200012 Health Risks Associated with Long-Term Finasteride and Dutasteride Use: It’s Time to Sound the Alarm Abdulmaged M. Traish Department of Urology, Boston University School of Medicine, Boston, MA, USA 5α-dihydrotestosterone (5α-DHT) is the most potent natural androgen. 5α-DHT elicits a multitude of physiological actions, in a host of tissues, including prostate, seminal vesicles, hair follicles, skin, kidney, and lacrimal and meibomian glands. How- ever, the physiological role of 5α-DHT in human physiology, remains questionable and, at best, poorly appreciated. Recent emerging literature supports a role for 5α-DHT in the physiological function of liver, pancreatic β-cell function and survival, ocular function and prevention of dry eye disease and kidney physiological function. Thus, inhibition of 5α-reductases with finasteride or dutasteride to reduce 5α-DHT biosynthesis in the course of treatment of benign prostatic hyperplasia (BPH) or male pattern hair loss, known as androgenetic alopecia (AGA) my induces a novel form of tissue specific androgen deficien- cy and contributes to a host of pathophysiological conditions, that are yet to be fully recognized. Here, we advance the con- cept that blockade of 5α-reductases by finasteride or dutasteride in a mechanism-based, irreversible, inhabitation of 5α-DHT biosynthesis results in a novel state of androgen deficiency, independent of circulating testosterone levels. Finasteride and dutasteride are frequently prescribed for long-term treatment of lower urinary tract symptoms in men with BPH and in men with AGA. -

Hormonal Treatment Strategies Tailored to Non-Binary Transgender Individuals
Journal of Clinical Medicine Review Hormonal Treatment Strategies Tailored to Non-Binary Transgender Individuals Carlotta Cocchetti 1, Jiska Ristori 1, Alessia Romani 1, Mario Maggi 2 and Alessandra Daphne Fisher 1,* 1 Andrology, Women’s Endocrinology and Gender Incongruence Unit, Florence University Hospital, 50139 Florence, Italy; [email protected] (C.C); jiska.ristori@unifi.it (J.R.); [email protected] (A.R.) 2 Department of Experimental, Clinical and Biomedical Sciences, Careggi University Hospital, 50139 Florence, Italy; [email protected]fi.it * Correspondence: fi[email protected] Received: 16 April 2020; Accepted: 18 May 2020; Published: 26 May 2020 Abstract: Introduction: To date no standardized hormonal treatment protocols for non-binary transgender individuals have been described in the literature and there is a lack of data regarding their efficacy and safety. Objectives: To suggest possible treatment strategies for non-binary transgender individuals with non-standardized requests and to emphasize the importance of a personalized clinical approach. Methods: A narrative review of pertinent literature on gender-affirming hormonal treatment in transgender persons was performed using PubMed. Results: New hormonal treatment regimens outside those reported in current guidelines should be considered for non-binary transgender individuals, in order to improve psychological well-being and quality of life. In the present review we suggested the use of hormonal and non-hormonal compounds, which—based on their mechanism of action—could be used in these cases depending on clients’ requests. Conclusion: Requests for an individualized hormonal treatment in non-binary transgender individuals represent a future challenge for professionals managing transgender health care. For each case, clinicians should balance the benefits and risks of a personalized non-standardized treatment, actively involving the person in decisions regarding hormonal treatment. -

Comparing the Effects of Combined Oral Contraceptives Containing Progestins with Low Androgenic and Antiandrogenic Activities on the Hypothalamic-Pituitary-Gonadal Axis In
JMIR RESEARCH PROTOCOLS Amiri et al Review Comparing the Effects of Combined Oral Contraceptives Containing Progestins With Low Androgenic and Antiandrogenic Activities on the Hypothalamic-Pituitary-Gonadal Axis in Patients With Polycystic Ovary Syndrome: Systematic Review and Meta-Analysis Mina Amiri1,2, PhD, Postdoc; Fahimeh Ramezani Tehrani2, MD; Fatemeh Nahidi3, PhD; Ali Kabir4, MD, MPH, PhD; Fereidoun Azizi5, MD 1Students Research Committee, School of Nursing and Midwifery, Department of Midwifery and Reproductive Health, Shahid Beheshti University of Medical Sciences, Tehran, Islamic Republic Of Iran 2Reproductive Endocrinology Research Center, Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Islamic Republic Of Iran 3School of Nursing and Midwifery, Department of Midwifery and Reproductive Health, Shahid Beheshti University of Medical Sciences, Tehran, Islamic Republic Of Iran 4Minimally Invasive Surgery Research Center, Iran University of Medical Sciences, Tehran, Islamic Republic Of Iran 5Endocrine Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Islamic Republic Of Iran Corresponding Author: Fahimeh Ramezani Tehrani, MD Reproductive Endocrinology Research Center Research Institute for Endocrine Sciences Shahid Beheshti University of Medical Sciences 24 Parvaneh Yaman Street, Velenjak, PO Box 19395-4763 Tehran, 1985717413 Islamic Republic Of Iran Phone: 98 21 22432500 Email: [email protected] Abstract Background: Different products of combined oral contraceptives (COCs) can improve clinical and biochemical findings in patients with polycystic ovary syndrome (PCOS) through suppression of the hypothalamic-pituitary-gonadal (HPG) axis. Objective: This systematic review and meta-analysis aimed to compare the effects of COCs containing progestins with low androgenic and antiandrogenic activities on the HPG axis in patients with PCOS. -

ORTHO TRI-CYCLEN® TABLETS ORTHO-CYCLEN® TABLETS (Norgestimate/Ethinyl Estradiol)
PHYSICIANS' PACKAGE INSERT ORTHO TRI-CYCLEN® TABLETS ORTHO-CYCLEN® TABLETS (norgestimate/ethinyl estradiol) Patients should be counseled that this product does not protect against HIV infection (AIDS) and other sexually transmitted diseases. DESCRIPTION Each of the following products is a combination oral contraceptive containing the progestational compound norgestimate and the estrogenic compound ethinyl estradiol. ORTHO TRI-CYCLEN 21 Tablets and ORTHO TRI-CYCLEN 28 Tablets. Each white tablet contains 0.180 mg of the progestational compound, norgestimate (18,19-Dinor- 17-pregn-4-en-20-yn-3-one,17-(acetyloxy)-13-ethyl-, oxime,(17α)-(+)-) and 0.035 mg of the estrogenic compound, ethinyl estradiol (19-nor-17α-pregna,1,3,5(10)-trien-20-yne-3,17-diol). Inactive ingredients include lactose, magnesium stearate, and pregelatinized starch. Each light blue tablet contains 0.215 mg of the progestational compound norgestimate (18,19- Dinor-17-pregn-4-en-20-yn-3-one,17-(acetyloxy)-13-ethyl-,oxime,(17α)-(+)-) and 0.035 mg of the estrogenic compound, ethinyl estradiol (19-nor-17α-pregna,1,3,5(10)-trien-20-yne-3,17-diol). Inactive ingredients include FD & C Blue No. 2 Aluminum Lake, lactose, magnesium stearate, and pregelatinized starch. Each blue tablet contains 0.250 mg of the progestational compound norgestimate (18,19-Dinor-17- pregn-4-en-20-yn-3-one, 17-(acetyloxy)-13-ethyl-,oxime,(17α)-(+)-) and 0.035 mg of the estrogenic compound, ethinyl estradiol (19-nor-17α-pregna,1,3,5(10)-trien-20-yne-3,17-diol). Inactive ingredients include FD & C Blue No. 2 Aluminum Lake, lactose, magnesium stearate, and pregelatinized starch. -

Estrogen and Progestin Hormone Doses in Combined Birth Control Pills
Estrogen and Progestin Hormone Doses in Combined Birth Control Pills Estrogen level Pill Brand Name Progestin Dose (mg) ethinyl estradiol (micrograms) 20 mcgm Alesse® levonorgestrel 0.10 Levlite® levonorgestrel 0.10 Loestrin 1/20® Fe norethindrone 1.00 acetate Mircette® desogestrel 0.15 Ortho Evra® norelgestromin 0.15 (patch) (norgestimate metabolite) phasic Estrostep® Fe norethindrone 1.0/1.0/1.0 20/30/35 mcgm acetate 30 mcgm Levlen® levonorgestrel 0.15 Levora® levonorgestrel 0.15 Nordette® levonorgestrel 0.15 Lo/Ovral® norgestrel 0.30 Desogen® desogestrel 0.15 Ortho-Cept® desogestrel 0.15 Loestrin® 1.5/30 norethindrone 1.50 acetate Yasmin® drospirenone 3.0 phasic Triphasil® levonorgestrel 0.05/0.075/0.125 30/40/30 mcgm Tri-Levlen® levonorgestrel 0.05/0.075/0.125 Trivora® levonorgestrel 0.05/0.075/0.125 35 mcgm Ortho-Cyclen® norgestimate 0.25 Ovcon-35® norethindrone 0.40 Brevicon® norethindrone 0.50 Modicon® norethindrone 0.50 Necon® norethindrone 1.00 Norethin® norethindrone 1.00 Norinyl® 1/35 norethindrone 1.00 Ortho-Novum® 1/35 norethindrone 1.00 Demulen® 1/35 ethynodiol diacetate 1.00 Zovia® 1/35E ethynodiol diacetate 1.00 phasic Ortho-Novum® norethindrone 0.50/1.00 35/35 mcgm 10/11 Jenest® norethindrone 0.50/1.00 phasic Ortho-Tri-Cyclen® norgestimate 0.15/0.215/0.25 35/35/35 mcgm Ortho-Novum® norethindrone 0.50/0.75/1.00 7/7/7 Tri-Norinyl® norethindrone 0.50/1.00/0.50 50 mcgm Necon® 1/50 norethindrone 1.00 Norinyl® 1/50 norethindrone 1.00 Ortho-Novum® 1/50 norethindrone 1.00 Ovcon-50® norethindrone 1.00 Ovral® norgestrel 0.50 Demulen® 1/50 ethynodiol diacetate 1.00 Zovia® 1/50E ethynodiol diacetate 1.00 Which pills have higher progestin side efects or cause more acne and hair growth? Each progestin has a diferent potency, milligram per milligram, in terms of progesterone efect to stop menstrual bleeding or androgen efect to stimulate acne and hair growth. -

Gender-Affirming Hormone Therapy
GENDER-AFFIRMING HORMONE THERAPY Julie Thompson, PA-C Medical Director of Trans Health, Fenway Health March 2020 fenwayhealth.org GOALS AND OBJECTIVES 1. Review process of initiating hormone therapy through the informed consent model 2. Provide an overview of masculinizing and feminizing hormone therapy 3. Review realistic expectations and benefits of hormone therapy vs their associated risks 4. Discuss recommendations for monitoring fenwayhealth.org PROTOCOLS AND STANDARDS OF CARE fenwayhealth.org WPATH STANDARDS OF CARE, 2011 The criteria for hormone therapy are as follows: 1. Well-documented, persistent (at least 6mo) gender dysphoria 2. Capacity to make a fully informed decision and to consent for treatment 3. Age of majority in a given country 4. If significant medical or mental health concerns are present, they must be reasonably well controlled fenwayhealth.org INFORMED CONSENT MODEL ▪ Requires healthcare provider to ▪ Effectively communicate benefits, risks and alternatives of treatment to patient ▪ Assess that the patient is able to understand and consent to the treatment ▪ Informed consent model does not preclude mental health care! ▪ Recognizes that prescribing decision ultimately rests with clinical judgment of provider working together with the patient ▪ Recognizes patient autonomy and empowers self-agency ▪ Decreases barriers to medically necessary care fenwayhealth.org INITIAL VISITS ▪ Review history of gender experience and patient’s goals ▪ Document prior hormone use ▪ Assess appropriateness for gender affirming medical -

Contraceptive Choices for Women with Inflammatory Bowel Disease
J Fam Plann Reprod Health Care: first published as 10.1783/147118903101197782 on 1 July 2003. Downloaded from Faculty of Family Planning and Reproductive Health Care Clinical Effectiveness Unit A unit funded by the FFPRHC and supported by the University of Aberdeen and SPCERH to provide guidance on evidence-based practice FFPRHC Guidance (July 2003) Contraceptive Choices for Women with Inflammatory Bowel Disease Journal of Family Planning and Reproductive Health Care 2003; 29(3): 127–135 This Guidance provides information on the effects of inflammatory bowel disease (IBD) in women of reproductive age with particular reference to contraceptive choices, fertility and pregnancy. For comprehensive advice on the management of patients with IBD, readers should refer to guidelines from the British Society for Gastroenterology.1 A key to the grades of recommendations, based on levels of evidence, is given at the end of this document. Details of the methods used by the Clinical Effectiveness Unit (CEU) in developing this Guidance, and evidence tables summarising the research basis of the recommendations, are available on the Faculty website (www.ffprhc.org.uk). Abbreviations used include: 5-aminosalicylic acid (5-ASA), body mass index (BMI), bone mineral density (BMD), combined oral contraception (COC), confidence interval (CI), copper-bearing intrauterine contraceptive device (IUD), Crohn’s disease (CD), depot medroxyprogesterone acetate (DMPA), dual X-ray absorptiometry (DEXA), emergency contraception (EC), gastrointestinal (GI), incidence rate ratio (IRR), inflammatory bowel disease (IBD), levonorgestrel-releasing intrauterine system (IUS), odds ratio (OR), progestogen-only emergency contraception (POEC), progestogen-only pill (POP), anti-tumour necrosis factor-alpha (anti- TNF-a), ulcerative colitis (UC), venous thromboembolism (VTE), World Health Organization (WHO). -
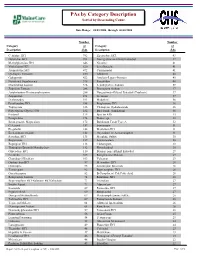
Report 752 by Category Description
PAs by Category Description Sorted by Descending Count Date Range: 04/01/2006 through 06/30/2006 Number Number Category of Category of Description PAs Description PAs Cetirizine HCl 792 Ziprasidone HCl 43 Duloxetine HCl 784 Norelgestromin-Ethinyl Estradiol 42 Methylphenidate HCl 646 Nicotine 41 Venlafaxine HCl 620 Levofloxacin 41 Atomoxetine HCl 472 Carisoprodol 41 Quetiapine Fumarate 430 Albuterol 40 Gabapentin 422 Amylase-Lipase-Protease 40 Nutritional Supplements 378 Famotidine 40 Montelukast Sodium 326 Levothyroxine Sodium 39 Zolpidem Tartrate 288 Enoxaparin Sodium 37 Amphetamine-Dextroamphetamine 286 Norgestimate-Ethinyl Estradiol (Triphasic) 37 Aripiprazole 271 Tretinoin 37 Desloratadine 191 Modafinil 36 Fexofenadine HCl 188 Pioglitazone HCl 36 Topiramate 186 Citalopram Hydrobromide 36 Polyethylene Glycol 3350 182 Budesonide (Inhalation) 36 Fentanyl 175 Epoetin Alfa 33 Eszopiclone 174 Etanercept 33 Esomeprazole Magnesium 172 Botulinum Toxin Type A 32 Celecoxib 157 Somatropin 31 Pregabalin 148 Metformin HCl 31 Escitalopram Oxalate 142 Oxycodone w/ Acetaminophen 31 Sertraline HCl 135 Morphine Sulfate 30 Risperidone 127 Levetiracetam 30 Bupropion HCl 118 Clonazepam 30 Tiotropium Bromide Monohydrate 112 Phenobarbital 30 Oxycodone HCl 110 Drospirenone-Ethinyl Estradiol 29 Ezetimibe 105 Rosiglitazone Maleate 29 Clopidogrel Bisulfate 103 Valsartan 29 Ondansetron HCl 97 Memantine HCl 28 Olanzapine 93 Sumatriptan Succinate 28 Temazepam 92 Buprenorphine HCl 28 Oxcarbazepine 82 B-Complex w/ C & Folic Acid 28 Rabeprazole Sodium 74 Ranitidine