USGS Professional Paper 1713, Chapter 31
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Having Regard to the Opinion of the European Chapter 1 of the Annex Binding Within Five Years of Parliament1 ; the Date of Entry Into Force of This Directive
878 Official Journal of the European Communities 29.10.71 Official Journal of the European Communities No L 243/29 COUNCIL DIRECTIVE of 18 October 1971 on the approximation of the laws of the Member States relating to units of measurement (71/354/EEC ) THE COUNCIL OF THE EUROPEAN COMMUNITIES, particular their names, symbols and use are not identical in the Member countries ; Having regard to the Treaty establishing the European Economic Community, and in particular HAS ADOPTED THIS DIRECTIVE : Article 100 thereof; Article 1 Having regard to the proposal from the Commission ; 1 . Member States shall make the provisions of Having regard to the Opinion of the European Chapter 1 of the Annex binding within five years of Parliament1 ; the date of entry into force of this Directive. 2 . Member States shall, with effect from 31 Having regard to the Opinion of the Economic and December 1977 at the latest, prohibit the use of the Social Committee2; units of measurement listed in Chapter III of the Annex. Whereas - the laws which regulate the use of units of measurement in the Member States differ from one 3 . The units of measurement temporarily" retained Member State to another and therefore hinder trade ; in accordance with the provisions of Chapter II or whereas application of the rules relating to measuring Chapter III of the Annex may not be brought into instruments is closely linked to the use of units of compulsory use by the Member States where they ' are measurement in the metrological system ; whereas, in not authorised at the date when this Directive enters into force . -

Guide for the Use of the International System of Units (SI)
Guide for the Use of the International System of Units (SI) m kg s cd SI mol K A NIST Special Publication 811 2008 Edition Ambler Thompson and Barry N. Taylor NIST Special Publication 811 2008 Edition Guide for the Use of the International System of Units (SI) Ambler Thompson Technology Services and Barry N. Taylor Physics Laboratory National Institute of Standards and Technology Gaithersburg, MD 20899 (Supersedes NIST Special Publication 811, 1995 Edition, April 1995) March 2008 U.S. Department of Commerce Carlos M. Gutierrez, Secretary National Institute of Standards and Technology James M. Turner, Acting Director National Institute of Standards and Technology Special Publication 811, 2008 Edition (Supersedes NIST Special Publication 811, April 1995 Edition) Natl. Inst. Stand. Technol. Spec. Publ. 811, 2008 Ed., 85 pages (March 2008; 2nd printing November 2008) CODEN: NSPUE3 Note on 2nd printing: This 2nd printing dated November 2008 of NIST SP811 corrects a number of minor typographical errors present in the 1st printing dated March 2008. Guide for the Use of the International System of Units (SI) Preface The International System of Units, universally abbreviated SI (from the French Le Système International d’Unités), is the modern metric system of measurement. Long the dominant measurement system used in science, the SI is becoming the dominant measurement system used in international commerce. The Omnibus Trade and Competitiveness Act of August 1988 [Public Law (PL) 100-418] changed the name of the National Bureau of Standards (NBS) to the National Institute of Standards and Technology (NIST) and gave to NIST the added task of helping U.S. -
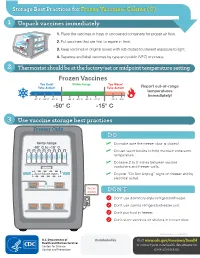
Storage Best Practices for Frozen Vaccines-Celsius
Storage Best Practices for Frozen Vaccines–Celsius (C) 1 Unpack vaccines immediately 1. Place the vaccines in trays or uncovered containers for proper air flow. 2. Put vaccines that are first to expire in front. HEP A - VFC 3. Keep vaccines in original boxes with lids closed to prevent exposure to light. 4. Separate and label vaccines by type and public (VFC) or private. 2 Thermostat should be at the factory-set or midpoint temperature setting Frozen Vaccines Too Cold! Within Range Too Warm! Take Action! Take Action! Report out-of-range temperatures immediately! -57° C -54° C -51° C -46° C -43° C -40° C -37° C -12° C -9°C -50° C -15° C 3 Use vaccine storage best practices Freezer Only DO temp range ✓ Do make sure the freezer door is closed! -50° C to -15° C ✓ Do use water bottles to help maintain consistent temperature. ✓ Do leave 2 to 3 inches between vaccine containers and freezer walls. don’t block vents ✓ Do post “Do Not Unplug” signs on freezer and by electrical outlet. do not unplug DON’T Don’t use dormitory-style refrigerator/freezer. Don’t use combo refrigerator/freezer unit. Don’t put food in freezer. Don’t store vaccines on shelves in freezer door. CS243541-D Revision %FDFNCFS 20 Distributed by Visit www.cdc.gov/vaccines/SandH or contact your state health department for more information. Test Your Knowledge 1 Which of the following units is the best for storing frozen vaccines? Freezer Freezer Freezer Freezer A. Full-size B. Full-size C. -
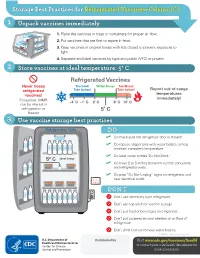
Storage Best Practices for Refrigerated Vaccines–Celsius (C)
Storage Best Practices for Refrigerated Vaccines–Celsius (C) 1 Unpack vaccines immediately 1. Place the vaccines in trays or containers for proper air flow. 2. Put vaccines that are first to expire in front. HEP A - VFC 3. Keep vaccines in original boxes with lids closed to prevent exposure to light. 4. Separate and label vaccines by type and public (VFC) or private. 2 Store vaccines at ideal temperature: 5° C Refrigerated Vaccines Never freeze Too Cold! Within Range Too Warm! refrigerated Take Action! Take Action! Report out-of-range vaccines! temperatures Exception: MMR immediately! -4˚ C -1˚ C 2˚ C 8˚ C 10˚ C can be stored in refrigerator or 5˚ C freezer 3 Use vaccine storage best practices Refrigerator Only DO ✓ Do make sure the refrigerator door is closed! ✓ Do replace crisper bins with water bottles to help maintain consistent temperature. ✓ Do label water bottles "Do Not Drink." 5° C ideal temp ✓ Do leave 2 to 3 inches between vaccine containers and refrigerator walls. ✓ Do post “Do Not Unplug” signs on refrigerator and do not near electrical outlet. unplug DON’T ; Don’t use dormitory-style refrigerator. ; Don’t use top shelf for vaccine storage. ; Don’t put food or beverages in refrigerator. ; Don’t put vaccines on door shelves or on floor of refrigerator. Don’t drink from or remove water bottles. ; CS243541-C Revision February 2018 Distributed by Visit www.cdc.gov/vaccines/SandH or contact your state health department for more information. Test Your Knowledge 1 Can you find at least 8 things that are wrong with vaccine storage in this refrigerator? 5° C ideal temp 2 When unpacking vaccines, why is it important to put the first-to-expire in the front? A. -
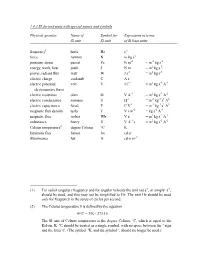
1.4.3 SI Derived Units with Special Names and Symbols
1.4.3 SI derived units with special names and symbols Physical quantity Name of Symbol for Expression in terms SI unit SI unit of SI base units frequency1 hertz Hz s-1 force newton N m kg s-2 pressure, stress pascal Pa N m-2 = m-1 kg s-2 energy, work, heat joule J N m = m2 kg s-2 power, radiant flux watt W J s-1 = m2 kg s-3 electric charge coulomb C A s electric potential, volt V J C-1 = m2 kg s-3 A-1 electromotive force electric resistance ohm Ω V A-1 = m2 kg s-3 A-2 electric conductance siemens S Ω-1 = m-2 kg-1 s3 A2 electric capacitance farad F C V-1 = m-2 kg-1 s4 A2 magnetic flux density tesla T V s m-2 = kg s-2 A-1 magnetic flux weber Wb V s = m2 kg s-2 A-1 inductance henry H V A-1 s = m2 kg s-2 A-2 Celsius temperature2 degree Celsius °C K luminous flux lumen lm cd sr illuminance lux lx cd sr m-2 (1) For radial (angular) frequency and for angular velocity the unit rad s-1, or simply s-1, should be used, and this may not be simplified to Hz. The unit Hz should be used only for frequency in the sense of cycles per second. (2) The Celsius temperature θ is defined by the equation θ/°C = T/K - 273.15 The SI unit of Celsius temperature is the degree Celsius, °C, which is equal to the Kelvin, K. -

The International System of Units (SI) - Conversion Factors For
NIST Special Publication 1038 The International System of Units (SI) – Conversion Factors for General Use Kenneth Butcher Linda Crown Elizabeth J. Gentry Weights and Measures Division Technology Services NIST Special Publication 1038 The International System of Units (SI) - Conversion Factors for General Use Editors: Kenneth S. Butcher Linda D. Crown Elizabeth J. Gentry Weights and Measures Division Carol Hockert, Chief Weights and Measures Division Technology Services National Institute of Standards and Technology May 2006 U.S. Department of Commerce Carlo M. Gutierrez, Secretary Technology Administration Robert Cresanti, Under Secretary of Commerce for Technology National Institute of Standards and Technology William Jeffrey, Director Certain commercial entities, equipment, or materials may be identified in this document in order to describe an experimental procedure or concept adequately. Such identification is not intended to imply recommendation or endorsement by the National Institute of Standards and Technology, nor is it intended to imply that the entities, materials, or equipment are necessarily the best available for the purpose. National Institute of Standards and Technology Special Publications 1038 Natl. Inst. Stand. Technol. Spec. Pub. 1038, 24 pages (May 2006) Available through NIST Weights and Measures Division STOP 2600 Gaithersburg, MD 20899-2600 Phone: (301) 975-4004 — Fax: (301) 926-0647 Internet: www.nist.gov/owm or www.nist.gov/metric TABLE OF CONTENTS FOREWORD.................................................................................................................................................................v -

Table of Contents
Table of Contents Teaching and Learning The Metric System Unit 1 1 - Suggested Teaching Sequence 1 - Objectives 1 - Rules of Notation 1 - Metric Units, Symbols, and Referents 2 - Metric Prefixes 2 - Linear Measurement Activities 3 - Area Measurement Activities 5 - Volume Measurement Activities 7 - Mass (Weight) Measurement Activities 9 - Temperature Measurement Activities 11 Unit 2 12 - Objectives 12 - Suggested Teaching Sequence 12 - Metrics in this Occupation 12 - Metric Units For Dietetic Technician 13 - Trying Out Metric Units 14 - Measuring With Metrics 15 Unit 3 16 - Objective 16 - Suggested Teaching Sequence 16 - Metric-Metric Equivalents 16 - Changing Units at Work 18 Unit 4 19 - Objective 19 - Suggested Teaching Sequence 19 - Selecting and Using Metric Instruments, Tools and Devices 19 - Which Tools for the Job? 20 - Measuring Up The Dietary Department 20 Unit 5 21 - Objective 21 - Suggested Teaching Sequence 21 - Metric-Customary Equivalents 21 - Conversion Table 22 - Any Way You Want It 23 Testing Metric Abilities 24 Answers to Exercises and Test 25 Temperature 26 Tools and Devices List References a::3CENTVI FOR VOCATlONAL I!DUCATION TEACHING AND LEARNING THE METRIC SYSTEM This metric instructional package was designed to meet job-related Unit 2 provide'S the metric terms which are used in this occupation metric measurement needs of students. To use this package students and gives experience with occupational measurement tasks. should already know the occupational terminology, measurement terms, and tools currently in use. These materials were prepared with Unit 3 focuses on job-related metric equivalents and their relation the help of experienced vocational teachers, reviewed by experts, tested ships. -
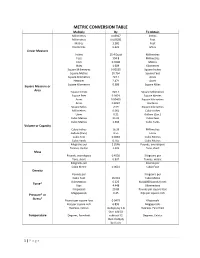
Metric Conversion Table
METRIC CONVERSION TABLE Multiply By To Obtain Millimetres 0.03937 Inches Millimetres 0.003281 Feet Metres 3.281 Feet Kilometres 0.621 Miles Linear Measure Inches 25.4 Exact Millimetres Feet 304.8 Millimetres Feet 0.3048 Metres Miles 1.609 Kilometres Square Millimetres 0.00155 Square Inches Square Metres 10.764 Square Feet Square Kilometres 247.1 Acres Hectares 2.471 Acres Square Kilometres 0.386 Square Miles Square Measure or Area Square Inches 645.2 Square Millimetres Square Feet 0.0929 Square Metres Acres 0.00405 Square Kilometres Acres 0.4047 Hectares Square Miles 2.59 Square Kilometres Millimetres 0.061 Cubic Inches Litres 0.22 Gallons (Can.) Cubic Metres 35.31 Cubic Feet Cubic Metres 1.308 Cubic Yards Volume or Capacity Cubic Inches 16.39 Millimetres Gallons (Can.) 4.55 Litres Cubic Feet 0.0283 Cubic Metres Cubic Yards 0.765 Cubic Metres Kilograms per 2.2046 Pounds, avoirdupois Tonnes, metric 1.102 Tons, short Mass Pounds, avoirdupois 0.4536 Kilograms per Tons, short 0.907 Tonnes, metric Kilograms per Pounds per Cubic Metre 0.0624 Cubic Foot Density Pounds per Kilograms per Cubic Foot 16.019 Cubic Metre Kilonewtons 0.225 Kips(1000 ponds force) Force* Kips 4.448 Kilonewtons Kilopascals 20.89 Pounds per square foot Megapascals 0.45 Kips per square inch Pressure* or Stress* Pounds per square foot 0.0479 Kilopascals Kips per square inch 6.895 Megapascals Degrees, Celsius multiply by 1.8 Degrees, Farenheit then add 32 Temperature Degrees, Farenheit subtract 32 Degrees, Celsius then multiply by 0.555 1 | P a g e METRIC CONVERSION GUIDE Linear Measurement One millimetre (1 mm) is equal to a thousandth part of a metre (0.001 m) and is a little greater than 1/32”. -

Kelvin Temperatures and Very Cold Things! 78
Kelvin Temperatures and Very Cold Things! 78 To keep track of some of the coldest things in the universe, scientists use the Kelvin temperature scale that begins at 0 Kelvin, which is also called Absolute Zero. Nothing can ever be colder than Absolute Zero because at this temperature, all motion stops. The table below shows some typical temperatures of different systems in the universe. Table of Cold Places and Things You are probably already familiar with the Celsius (C) and Fahrenheit (F) Temp. Object or Event temperature scales. The two formulas (K) below show how to switch from degrees- 183 Vostok, Antarctica C to degrees-F. 160 Phobos- a moon of mars 5 9 134 Superconductors C = --- ( F - 32 ) F = --- C + 32 128 Europa in the summer 9 5 120 Moon at night 95 Titan surface temp. Because the Kelvin scale is related to 90 Liquid oxygen the Celsius scale, we can also convert 88 Miranda surface temp. from Celsius to Kelvin (K) using the 81 Enceladus in the summer equation: 77 Liquid nitrogen 70 Mercury at night K = 273 + C 63 Solid nitrogen 55 Pluto in the summertime Use these three equations to convert 54 Solid oxygen between the three temperature scales: 50 Dwarf Planet Quaoar Problem 1: 212 F converted to K 45 Shadowed crater on moon 40 Star-forming nebula Problem 2: 0 K converted to F 33 Pluto in the wintertime 20 Liquid nitrogen Problem 3: 100 C converted to K 19 Bose-Einstein condensate 4 Liquid helium Problem 4: -150 F converted to K 3 Cosmic background light 2 Liquid helium Problem 5: -150 C converted to K 1 Boomerang Nebula 0 ABSOLUTE ZERO Problem 6: Two scientists measure the daytime temperature of the moon using two different instruments. -

Mobius Units Help SI/Metric Units (Accept Prefixes)
Mobius Units Help The Unit column of the following tables display all of the units that are recognized by the system. You can use either the units themselves, or combinations of these units, for example kJ/mol, kg*m^2, or m/s/s. The system accepts equivalent answers with different units as long as both units are accepted in the system. That is, if the answer is 120 cm, then 1.2 m or 1200 mm will also be accepted as correct. SI/Metric Units (Accept Prefixes) The units in this section can each be prefixed with one of the accepted SI prefixes below. SI Prefixes Base Units Prefix Factor Name Unit Definition Name Y 10^24 yotta m meter Z 10^21 zetta s second E 10^18 exa kg kilogram P 10^15 peta A amp T 10^12 tera K kelvin G 10^9 giga M 10^6 mega Derived SI Units k 10^3 kilo Unit Definition Name 6.02214199 * h 10^2 hecto mol mole da 10^1 deca 10^23 d 10^-1 deci rad 1 radian c 10^-2 centi sr 1 steradian m 10^-3 milli Hz 1/s hertz u 10^-6 micro N kg m/s^2 newton n 10^-9 nano Pa N/m^2 pascal p 10^-12 pico J N m joule f 10^-15 femto W J/s watt a 10^-18 atto C A s coulomb z 10^-21 zepto V J/C volt y 10^-24 yocto F C/V farad ohm V/A ohm CGS Units S A/V siemens Unit Definition Name Wb V s weber erg 10^-7 J erg T Wb/m^2 tesla dyn 10^-5 N dyne H Wb/A henry P 0.1 Pa*s poise lm cd sr lumen St cm^2/s stokes lx lm/m^2 lux sb cd/cm^2 stilb Bq s^-1 becquerel ph 10^4 lx phot Gy J/kg gray Gal cm/s^2 gal Sv J/kg sievert Mx 10^-8 Wb maxwell kat mol/s katal G 10^-4 T gauss Metric Units oersted Oe (10^3/4/Pi) A/m (rationalized) Unit Definition Name L m^3/1000 liter M mol/L molar eV J*1.602E-19 electron volt SI/Metric Units (No Prefixes) These units, and alternative ways of writing units, do not accept SI prefixes. -

Scald Injury Prevention Educator's Guide
American Burn Association SCALD INJURY PREVENTION Educator’s Guide A Community Fire and Burn Prevention Program Supported by the United States Fire Administration Federal Emergency Management Agency General Background Information on Scald Burns • High risk groups • Time and temperature relationship • Selected burn and scald injury statistics for children Fact Sheets for Community Distribution • Hot food and beverage scalds • Microwave scald prevention • Tap water scald prevention • Water heater thermostat settings and scald prevention • Other causes of scald burns Getting the Message to the Media • Sample press releases • Sample Public Service Announcements References/Resources PowerPoint Slide Presentation with Instructor Comments Evaluation Form Community Fire & Burn Prevention Programs Scald Injury Prevention Campaign GENERAL BACKGROUND INFORMATION ON SCALD BURNS Although scald burns can happen to anyone, young children, older adults and people with disabilities are the most likely to incur such injuries. Most scald burn injuries happen in the home, in connection with the preparation or serving of hot food or beverages, or from exposure to hot tap water in bathtubs or showers. Severe scalds also occur in the workplace, typically when pipes or valves fail while carrying or regulating the flow of steam. Both behavioral and environmental measures may be needed to protect those vulnerable to scalds because of age or disability, or because they do not have control of the hot water temperature in multi-unit residential buildings. The severity of a scald injury depends on the temperature to which the skin is exposed and how long it is exposed. The most common regulatory standard for the maximum temperature of water delivered by residential water heaters to the tap is 120 degrees Fahrenheit/48 degrees Celsius. -
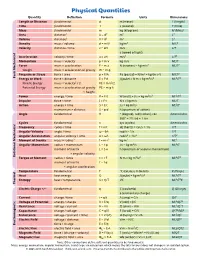
Physical Quantities
Physical Quantities Quantity Definition Formula Units Dimensions Length or Distance fundamental d m (meter) L (Length) Time fundamental t s (second) T (Time) Mass fundamental m kg (kilogram) M (Mass) Area distance2 A = d2 m2 L2 Volume distance3 V = d3 m3 L3 Density mass / volume d = m/V kg/m3 M/L3 Velocity distance / time v = d/t m/s L/T c (speed of light) Acceleration velocity / time a = v/t m/s2 L/T2 Momentum mass × velocity p = m·v kg·m/s ML/T Force mass × acceleration F = m·a N (newton) = kg·m/s2 ML/T2 Weight mass × acceleration of gravity W = m·g Pressure or Stress force / area p = F/A Pa (pascal) = N/m2 = kg/(m·s2) M/LT2 Basic Mechanical Energy or Work force × distance E = F·d J (joule) = N·m = kg·m2/s2 ML2/T2 Kinetic Energy mass × velocity2 / 2 KE = m·v2/2 Potential Energy mass × acceleration of gravity PE = m·g·h × height Power energy / time P = E/t W (watt) = J/s = kg·m2/s3 ML2/T3 Impulse force × time I = F·t N·s = kg·m/s ML/T Action energy × time S = E·t J·s = kg·m2/s ML2/T momentum × distance S = p·d h (quantum of action) Angle fundamental θ ° (degree), rad (radian), rev dimensionless 360° = 2π rad = 1 rev Cycles fundamental n cyc (cycles) dimensionless Frequency cycles / time f = n/t Hz (hertz) = cyc/s = 1/s 1/T Angular Velocity angle / time ω = θ/t rad/s = 1/s 1/T Angular Acceleration angular velocity / time α = ω/t rad/s2 = 1/s2 1/T2 Moment of Inertia mass × radius2 I = m·r2 kg·m2 ML2 Angular Momentum radius × momentum L = r·p J·s = kg·m2/s ML2/T moment of inertia L = I·ω ħ (quantum of angular momentum) × angular velocity