4 Converting Degrees Celsius to Degrees Fahrenheit
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Having Regard to the Opinion of the European Chapter 1 of the Annex Binding Within Five Years of Parliament1 ; the Date of Entry Into Force of This Directive
878 Official Journal of the European Communities 29.10.71 Official Journal of the European Communities No L 243/29 COUNCIL DIRECTIVE of 18 October 1971 on the approximation of the laws of the Member States relating to units of measurement (71/354/EEC ) THE COUNCIL OF THE EUROPEAN COMMUNITIES, particular their names, symbols and use are not identical in the Member countries ; Having regard to the Treaty establishing the European Economic Community, and in particular HAS ADOPTED THIS DIRECTIVE : Article 100 thereof; Article 1 Having regard to the proposal from the Commission ; 1 . Member States shall make the provisions of Having regard to the Opinion of the European Chapter 1 of the Annex binding within five years of Parliament1 ; the date of entry into force of this Directive. 2 . Member States shall, with effect from 31 Having regard to the Opinion of the Economic and December 1977 at the latest, prohibit the use of the Social Committee2; units of measurement listed in Chapter III of the Annex. Whereas - the laws which regulate the use of units of measurement in the Member States differ from one 3 . The units of measurement temporarily" retained Member State to another and therefore hinder trade ; in accordance with the provisions of Chapter II or whereas application of the rules relating to measuring Chapter III of the Annex may not be brought into instruments is closely linked to the use of units of compulsory use by the Member States where they ' are measurement in the metrological system ; whereas, in not authorised at the date when this Directive enters into force . -

Water Heater Formulas and Terminology
More resources http://waterheatertimer.org/9-ways-to-save-with-water-heater.html http://waterheatertimer.org/Figure-Volts-Amps-Watts-for-water-heater.html http://waterheatertimer.org/pdf/Fundamentals-of-water-heating.pdf FORMULAS & FACTS BTU (British Thermal Unit) is the heat required to raise 1 pound of water 1°F 1 BTU = 252 cal = 0.252 kcal 1 cal = 4.187 Joules BTU X 1.055 = Kilo Joules BTU divided by 3,413 = Kilowatt (1 KW) FAHRENHEIT CENTIGRADE 32 0 41 5 To convert from Fahrenheit to Celsius: 60.8 16 (°F – 32) x 5/9 or .556 = °C. 120.2 49 140 60 180 82 212 100 One gallon of 120°F (49°C) water BTU output (Electric) = weighs approximately 8.25 pounds. BTU Input (Not exactly true due Pounds x .45359 = Kilogram to minimal flange heat loss.) Gallons x 3.7854 = Liters Capacity of a % of hot water = cylindrical tank (Mixed Water Temp. – Cold Water – 1⁄ 2 diameter (in inches) Temp.) divided by (Hot Water Temp. x 3.146 x length. (in inches) – Cold Water Temp.) Divide by 231 for gallons. % thermal efficiency = Doubling the diameter (GPH recovery X 8.25 X temp. rise X of a pipe will increase its flow 1.0) divided by BTU/H Input capacity (approximately) 5.3 times. BTU output (Gas) = GPH recovery x 8.25 x temp. rise x 1.0 FORMULAS & FACTS TEMP °F RISE STEEL COPPER Linear expansion of pipe 50° 0.38˝ 0.57˝ – in inches per 100 Ft. 100° .076˝ 1.14˝ 125° .092˝ 1.40˝ 150° 1.15˝ 1.75˝ Grain – 1 grain per gallon = 17.1 Parts Per million (measurement of water hardness) TC-092 FORMULAS & FACTS GPH (Gas) = One gallon of Propane gas contains (BTU/H Input X % Eff.) divided by about 91,250 BTU of heat. -

How Does the Thermometer Work?
How Does the Thermometer Work? How Does the Thermometer Work? A thermometer is a device that measures the temperature of things. The name is made up of two smaller words: "Thermo" means heat and "meter" means to measure. You can use a thermometer to tell the temperature outside or inside your house, inside your oven, even the temperature of your body if you're sick. One of the earliest inventors of a thermometer was probably Galileo. We know him more for his studies about the solar system and his "revolutionary" theory (back then) that the earth and planets rotated around the sun. Galileo is said to have used a device called a "thermoscope" around 1600 - that's 400 years ago!! The thermometers we use today are different than the ones Galileo may have used. There is usually a bulb at the base of the thermometer with a long glass tube stretching out the top. Early thermometers used water, but because water freezes there was no way to measure temperatures less than the freezing point of water. So, alcohol, which freezes at temperature below the point where water freezes, was used. The red colored or silver line in the middle of the thermometer moves up and down depending on the temperature. The thermometer measures temperatures in Fahrenheit, Celsius and another scale called Kelvin. Fahrenheit is used mostly in the United States, and most of the rest of the world uses Celsius. Kelvin is used by scientists. Fahrenheit is named after the German physicist Gabriel D. Fahrenheit who developed his scale in 1724. -

Guide for the Use of the International System of Units (SI)
Guide for the Use of the International System of Units (SI) m kg s cd SI mol K A NIST Special Publication 811 2008 Edition Ambler Thompson and Barry N. Taylor NIST Special Publication 811 2008 Edition Guide for the Use of the International System of Units (SI) Ambler Thompson Technology Services and Barry N. Taylor Physics Laboratory National Institute of Standards and Technology Gaithersburg, MD 20899 (Supersedes NIST Special Publication 811, 1995 Edition, April 1995) March 2008 U.S. Department of Commerce Carlos M. Gutierrez, Secretary National Institute of Standards and Technology James M. Turner, Acting Director National Institute of Standards and Technology Special Publication 811, 2008 Edition (Supersedes NIST Special Publication 811, April 1995 Edition) Natl. Inst. Stand. Technol. Spec. Publ. 811, 2008 Ed., 85 pages (March 2008; 2nd printing November 2008) CODEN: NSPUE3 Note on 2nd printing: This 2nd printing dated November 2008 of NIST SP811 corrects a number of minor typographical errors present in the 1st printing dated March 2008. Guide for the Use of the International System of Units (SI) Preface The International System of Units, universally abbreviated SI (from the French Le Système International d’Unités), is the modern metric system of measurement. Long the dominant measurement system used in science, the SI is becoming the dominant measurement system used in international commerce. The Omnibus Trade and Competitiveness Act of August 1988 [Public Law (PL) 100-418] changed the name of the National Bureau of Standards (NBS) to the National Institute of Standards and Technology (NIST) and gave to NIST the added task of helping U.S. -

Multidisciplinary Design Project Engineering Dictionary Version 0.0.2
Multidisciplinary Design Project Engineering Dictionary Version 0.0.2 February 15, 2006 . DRAFT Cambridge-MIT Institute Multidisciplinary Design Project This Dictionary/Glossary of Engineering terms has been compiled to compliment the work developed as part of the Multi-disciplinary Design Project (MDP), which is a programme to develop teaching material and kits to aid the running of mechtronics projects in Universities and Schools. The project is being carried out with support from the Cambridge-MIT Institute undergraduate teaching programe. For more information about the project please visit the MDP website at http://www-mdp.eng.cam.ac.uk or contact Dr. Peter Long Prof. Alex Slocum Cambridge University Engineering Department Massachusetts Institute of Technology Trumpington Street, 77 Massachusetts Ave. Cambridge. Cambridge MA 02139-4307 CB2 1PZ. USA e-mail: [email protected] e-mail: [email protected] tel: +44 (0) 1223 332779 tel: +1 617 253 0012 For information about the CMI initiative please see Cambridge-MIT Institute website :- http://www.cambridge-mit.org CMI CMI, University of Cambridge Massachusetts Institute of Technology 10 Miller’s Yard, 77 Massachusetts Ave. Mill Lane, Cambridge MA 02139-4307 Cambridge. CB2 1RQ. USA tel: +44 (0) 1223 327207 tel. +1 617 253 7732 fax: +44 (0) 1223 765891 fax. +1 617 258 8539 . DRAFT 2 CMI-MDP Programme 1 Introduction This dictionary/glossary has not been developed as a definative work but as a useful reference book for engi- neering students to search when looking for the meaning of a word/phrase. It has been compiled from a number of existing glossaries together with a number of local additions. -
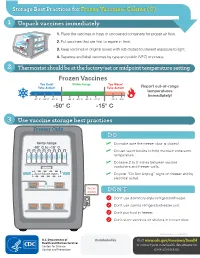
Storage Best Practices for Frozen Vaccines-Celsius
Storage Best Practices for Frozen Vaccines–Celsius (C) 1 Unpack vaccines immediately 1. Place the vaccines in trays or uncovered containers for proper air flow. 2. Put vaccines that are first to expire in front. HEP A - VFC 3. Keep vaccines in original boxes with lids closed to prevent exposure to light. 4. Separate and label vaccines by type and public (VFC) or private. 2 Thermostat should be at the factory-set or midpoint temperature setting Frozen Vaccines Too Cold! Within Range Too Warm! Take Action! Take Action! Report out-of-range temperatures immediately! -57° C -54° C -51° C -46° C -43° C -40° C -37° C -12° C -9°C -50° C -15° C 3 Use vaccine storage best practices Freezer Only DO temp range ✓ Do make sure the freezer door is closed! -50° C to -15° C ✓ Do use water bottles to help maintain consistent temperature. ✓ Do leave 2 to 3 inches between vaccine containers and freezer walls. don’t block vents ✓ Do post “Do Not Unplug” signs on freezer and by electrical outlet. do not unplug DON’T Don’t use dormitory-style refrigerator/freezer. Don’t use combo refrigerator/freezer unit. Don’t put food in freezer. Don’t store vaccines on shelves in freezer door. CS243541-D Revision %FDFNCFS 20 Distributed by Visit www.cdc.gov/vaccines/SandH or contact your state health department for more information. Test Your Knowledge 1 Which of the following units is the best for storing frozen vaccines? Freezer Freezer Freezer Freezer A. Full-size B. Full-size C. -
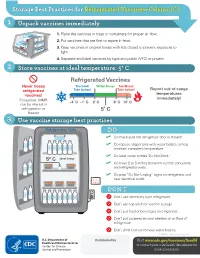
Storage Best Practices for Refrigerated Vaccines–Celsius (C)
Storage Best Practices for Refrigerated Vaccines–Celsius (C) 1 Unpack vaccines immediately 1. Place the vaccines in trays or containers for proper air flow. 2. Put vaccines that are first to expire in front. HEP A - VFC 3. Keep vaccines in original boxes with lids closed to prevent exposure to light. 4. Separate and label vaccines by type and public (VFC) or private. 2 Store vaccines at ideal temperature: 5° C Refrigerated Vaccines Never freeze Too Cold! Within Range Too Warm! refrigerated Take Action! Take Action! Report out-of-range vaccines! temperatures Exception: MMR immediately! -4˚ C -1˚ C 2˚ C 8˚ C 10˚ C can be stored in refrigerator or 5˚ C freezer 3 Use vaccine storage best practices Refrigerator Only DO ✓ Do make sure the refrigerator door is closed! ✓ Do replace crisper bins with water bottles to help maintain consistent temperature. ✓ Do label water bottles "Do Not Drink." 5° C ideal temp ✓ Do leave 2 to 3 inches between vaccine containers and refrigerator walls. ✓ Do post “Do Not Unplug” signs on refrigerator and do not near electrical outlet. unplug DON’T ; Don’t use dormitory-style refrigerator. ; Don’t use top shelf for vaccine storage. ; Don’t put food or beverages in refrigerator. ; Don’t put vaccines on door shelves or on floor of refrigerator. Don’t drink from or remove water bottles. ; CS243541-C Revision February 2018 Distributed by Visit www.cdc.gov/vaccines/SandH or contact your state health department for more information. Test Your Knowledge 1 Can you find at least 8 things that are wrong with vaccine storage in this refrigerator? 5° C ideal temp 2 When unpacking vaccines, why is it important to put the first-to-expire in the front? A. -

Temperature Scales (Celsius Vs Fahrenheit)
TEMPERATURE SCALES (CELSIUS VS FAHRENHEIT) The Celsius (also known as centigrade) temperature scale is the overwhelmingly popular scale around the world. Americans are really the only folks stubborn enough to hang on to our old habits. Anders Celsius, (1701 – 1744) born in Uppsala Sweden, was one of a large number of scientists (all related) originating from Ovanåker in the province of Hälsingland. The family name is a latinised version of the name of the vicarage (Högen). His grandfathers were both professors in Uppsala: Magnus Celsius the mathematician and Anders Spole the astronomer. His father, Nils Celsius, was also professor in astronomy. Celsius, who was said to have been very talented in mathematics from an early age, was appointed professor of astronomy in 1730. For his metereological observations he constructed his world famous Celsius thermometer, with 0 for the boiling point of water and 100 for the freezing point. After his death in 1744 the scale was reversed to its present form. http://www.astro.uu.se/history/Celsius_eng.html What can be considered the first modern thermometer, the mercury thermometer with a standardized scale, was invented by Daniel Gabriel Fahrenheit (1686-1736) in 1714. The Fahrenheit scale divided the freezing and boiling points of water into 180 degrees. 32°F was the freezing p0int of water and 212°F was the boiling point of water. 0°F was based on the temperature of an equal mixture of water, ice, and salt. Daniel Fahrenheit based his temperature scale on the temperature of the human body. Originally, the human body temperature was 100° F on the Fahrenheit scale, but it has since been adjusted to 98.6°F. -

Metric System Conversion Factors1 J
AGR39 Metric System Conversion Factors1 J. Bryan Unruh, Barry J. Brecke, and Ramon G. Leon-Gonzalez2 Area Equivalents 1 Hectare (ha) 2 1 Acre (A) = 10,000 square meters (m ) 2 = 100 are (a) = 43,560 square feet (ft ) = 2.471 acres (A) = 4,840 square yards (yd2) = 0.405 hectares (ha) 1 Square Foot (ft) = 160 square rods (rd2) 2 = 4,047 square meters (m2) = 144 square inches (in ) = 929.03 square centimeters (cm2) 2 1 Acre-inch (ac-in) = 0.0929 square meters (m ) 3 = 102.8 cubic meters (m ) 1 Square Mile (mi) = 27,154 gallons, US (gal) 2 = 3,630 cubic feet (ft3) = 27,878,400 square feet (ft ) = 3,097,600 square yards (yd2) 2 1 Are (a) = 640 square acres (A ) = 2,589,988.11 square meters (m2) = 100 square meters (m2) 2 = 119.6 square yards (yd ) 1 Square Rod (rd) = 0.025 acre (A) = 39,204 square inches (in2) = 272.25 square feet (ft2) 1 Cubic Foot (ft) 2 3 = 30.25 square yards (yds ) = 1,728 cubic inches (in ) = 25.3 square meters (m2) = 0.037 cubic yards (yds3) 3 = 0.02832 cubic meters (cm ) 1 Square Yard (yd) = 28,320 cubic centimeters (cm3) = 9 square feet (ft2) 2 1 Cubic Yard (yd) = 0.836 square meters (m ) = 27 cubic feet (ft3) = 0.764 cubic meters (m3) 1. This document is AGR39, one of a series of the Environmental Horticulture Department, UF/IFAS Extension. Original publication date November 1993. Revised December 2014. Reviewed December 2017. Visit the EDIS website at http://edis.ifas.ufl.edu. -

Enghandbook.Pdf
785.392.3017 FAX 785.392.2845 Box 232, Exit 49 G.L. Huyett Expy Minneapolis, KS 67467 ENGINEERING HANDBOOK TECHNICAL INFORMATION STEELMAKING Basic descriptions of making carbon, alloy, stainless, and tool steel p. 4. METALS & ALLOYS Carbon grades, types, and numbering systems; glossary p. 13. Identification factors and composition standards p. 27. CHEMICAL CONTENT This document and the information contained herein is not Quenching, hardening, and other thermal modifications p. 30. HEAT TREATMENT a design standard, design guide or otherwise, but is here TESTING THE HARDNESS OF METALS Types and comparisons; glossary p. 34. solely for the convenience of our customers. For more Comparisons of ductility, stresses; glossary p.41. design assistance MECHANICAL PROPERTIES OF METAL contact our plant or consult the Machinery G.L. Huyett’s distinct capabilities; glossary p. 53. Handbook, published MANUFACTURING PROCESSES by Industrial Press Inc., New York. COATING, PLATING & THE COLORING OF METALS Finishes p. 81. CONVERSION CHARTS Imperial and metric p. 84. 1 TABLE OF CONTENTS Introduction 3 Steelmaking 4 Metals and Alloys 13 Designations for Chemical Content 27 Designations for Heat Treatment 30 Testing the Hardness of Metals 34 Mechanical Properties of Metal 41 Manufacturing Processes 53 Manufacturing Glossary 57 Conversion Coating, Plating, and the Coloring of Metals 81 Conversion Charts 84 Links and Related Sites 89 Index 90 Box 232 • Exit 49 G.L. Huyett Expressway • Minneapolis, Kansas 67467 785-392-3017 • Fax 785-392-2845 • [email protected] • www.huyett.com INTRODUCTION & ACKNOWLEDGMENTS This document was created based on research and experience of Huyett staff. Invaluable technical information, including statistical data contained in the tables, is from the 26th Edition Machinery Handbook, copyrighted and published in 2000 by Industrial Press, Inc. -

Thermometer ×
This website would like to remind you: Your browser (Apple Safari 4) is out of date. Update your browser for more × security, comfort and the best experience on this site. Encyclopedic Entry thermometer For the complete encyclopedic entry with media resources, visit: http://education.nationalgeographic.com/encyclopedia/thermometer/ A thermometer is an instrument that measures temperature. It can measure the temperature of a solid such as food, a liquid such as water, or a gas such as air. The three most common units of measurement for temperature are Celsius, Fahrenheit, and kelvin. The Celsius scale is part of the metric system. The metric system of measurement also includes units of mass, such as kilograms, and units of length, such as kilometers. The metric system, including Celsius, is the official system of measurement for almost all countries in the world. Most scientific fields measure temperature using the Celsius scale. Zero degrees Celsius is the freezing point of water, and 100 degrees Celsius is the boiling point of water. Three nations do not use the Celsius scale. The United States, Burma, and Liberia use the Fahrenheit scale to measure temperature. However, even in these countries, scientists use the Celsius or kelvin scale to measure temperature. Water freezes at 32 degrees Fahrenheit and boils at 212 degrees Fahrenheit. The Kelvin scale is used by physicists and other scientists who need to record very precise temperatures. The kelvin scale is the only unit of measurement to include the temperature for "absolute zero," the total absence of any heat energy. This makes the kelvin scale essential to scientists who calculate the temperature of objects in the cold reaches of outer space. -
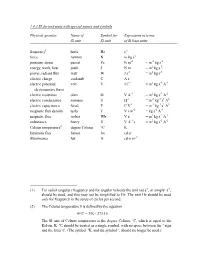
1.4.3 SI Derived Units with Special Names and Symbols
1.4.3 SI derived units with special names and symbols Physical quantity Name of Symbol for Expression in terms SI unit SI unit of SI base units frequency1 hertz Hz s-1 force newton N m kg s-2 pressure, stress pascal Pa N m-2 = m-1 kg s-2 energy, work, heat joule J N m = m2 kg s-2 power, radiant flux watt W J s-1 = m2 kg s-3 electric charge coulomb C A s electric potential, volt V J C-1 = m2 kg s-3 A-1 electromotive force electric resistance ohm Ω V A-1 = m2 kg s-3 A-2 electric conductance siemens S Ω-1 = m-2 kg-1 s3 A2 electric capacitance farad F C V-1 = m-2 kg-1 s4 A2 magnetic flux density tesla T V s m-2 = kg s-2 A-1 magnetic flux weber Wb V s = m2 kg s-2 A-1 inductance henry H V A-1 s = m2 kg s-2 A-2 Celsius temperature2 degree Celsius °C K luminous flux lumen lm cd sr illuminance lux lx cd sr m-2 (1) For radial (angular) frequency and for angular velocity the unit rad s-1, or simply s-1, should be used, and this may not be simplified to Hz. The unit Hz should be used only for frequency in the sense of cycles per second. (2) The Celsius temperature θ is defined by the equation θ/°C = T/K - 273.15 The SI unit of Celsius temperature is the degree Celsius, °C, which is equal to the Kelvin, K.