3.2 Periodic Trends Mrs
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
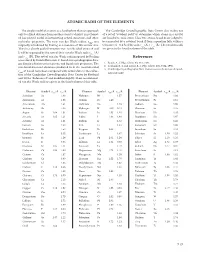
ATOMIC RADII of the ELEMENTS References
ATOMIC RADII OF THE ElEMENTS The simple model of an atom as a hard sphere that can approach The Cambridge Crystallographic Data Center also makes use only to a fixed distance from another atom to which it is not bond- of a set of “covalent radii” to determine which atoms in a crystal ed has proved useful in interpreting crystal structures and other are bonded to each other . Thus two atoms A and B are judged to molecular properties . The term van der Waals radius, rvdw, was be connected by a covalent bond if their separation falls within a originally introduced by Pauling as a measure of this atomic size . tolerance of ±0 .4 Å of the sum rcov (A) + rcov (B) . The covalent radii Thus in a closely packed structure two non-bonded atoms A and are given in the fourth column of the table . B will be separated by the sum of their van der Waals radii rvdw (A) and rvdw (B) . The set of van der Waals radii proposed by Pauling References was refined by Bondi (Reference 1) based on crystallographic data, gas kinetic collision cross sections, and liquid state properties . The 1 . Bondi, A ., J. Phys. Chem. 68, 441, 1964 . non-bonded contact distances predicted from the recommended 2 . Rowland, R . S . and Taylor, R ., J. Phys. Chem. 100, 7384, 1996 . 3 . Cambridge Crystallographic Data Center, www .ccdc .cam .ac .uk/prod- r of Bondi have been compared with actual data in the collec- vdw ucts/csd/radii/ tion of the Cambridge Crystallographic Data Center by Rowland and Taylor (Reference 2) and modified slightly . -

Modeling the Shape of Ions in Pyrite-Type Crystals
Crystals 2014, 4, 390-403; doi:10.3390/cryst4030390 OPEN ACCESS crystals ISSN 2073-4352 www.mdpi.com/journal/crystals Article Modeling the Shape of Ions in Pyrite-Type Crystals Mario Birkholz IHP, Im Technologiepark 25, 15236 Frankfurt (Oder), Germany; E-Mail: [email protected]; Tel.: +49-335-56250 Received: 13 April 2014; in revised form: 22 August 2014 / Accepted: 26 August 2014 / Published: 3 September 2014 Abstract: The geometrical shape of ions in crystals and the concept of ionic radii are re-considered. The re-investigation is motivated by the fact that a spherical modelling is justified for p valence shell ions on cubic lattice sites only. For the majority of point groups, however, the ionic radius must be assumed to be an anisotropic quantity. An appropriate modelling of p valence ions then has to be performed by ellipsoids. The approach is tested for pyrite-structured dichalcogenides MX2, with chalcogen ions X = O, S, Se and Te. The latter are found to exhibit the shape of ellipsoids being compressed along the <111> symmetry axes, with two radii r|| and describing their spatial extension. Based on this ansatz, accurate interatomic M–X distances can be derived and a consistent geometrical model emerges for pyrite-structured compounds. Remarkably, the volumes of chalcogen ions are found to vary only little in different MX2 compounds, suggesting the ionic volume rather than the ionic radius to behave as a crystal-chemical constant. Keywords: ionic radius; ionic shape; bonding distance; ionic volume; pyrite-type compounds; di-chalcogenides; di-oxides; di-sulfides; di-selenides; di-tellurides 1. -

A Study of the Hydration of the Alkali Metal Ions in Aqueous Solution
Article pubs.acs.org/IC A Study of the Hydration of the Alkali Metal Ions in Aqueous Solution Johan Mahler̈ and Ingmar Persson* Department of Chemistry, Swedish University of Agricultural Sciences, P.O. Box 7015, SE-750 07 Uppsala, Sweden *S Supporting Information ABSTRACT: The hydration of the alkali metal ions in aqueous solution has been studied by large angle X-ray scattering (LAXS) and double difference infrared spectroscopy (DDIR). The structures of the dimethyl sulfoxide solvated alkali metal ions in solution have been determined to support the studies in aqueous solution. The results of the LAXS and DDIR mea- surements show that the sodium, potassium, rubidium and cesium ions all are weakly hydrated with only a single shell of water molecules. The smaller lithium ion is more strongly hydrated, most probably with a second hydration shell present. The influence of the rubidium and cesium ions on the water structure was found to be very weak, and it was not possible to quantify this effect in a reliable way due to insufficient separation of the O−D stretching bands of partially deuterated water bound to these metal ions and the O−D stretching bands of the bulk water. Aqueous solutions of sodium, potassium and cesium iodide and cesium and lithium hydroxide have been studied by LAXS and M−O bond distances have been determined fairly accurately except for lithium. However, the number of water molecules binding to the alkali metal ions is very difficult to determine from the LAXS measurements as the number of distances and the temperature factor are strongly correlated. -

CHEM 1A03 UNIT 3: Periodic Trends Introduction
CHEM 1A03 UNIT 3: Periodic Trends Introduction Hey! Thanks for opening up this Chemistry Periodic Trends Review! The education team at WebStraw McMaster has put together a comprehensive breakdown for you that covers all the key concepts for Chemistry Periodic Trends Members of our team have taken the course in previous years, and we understand better than anyone else what specific ideas and concepts tend to trip students up throughout the semester. We are essentially offering you the key takeaways from the course, after having completed the course ourselves. Before you read further, also keep in mind that these review packages are not meant to be a tool for you to learn the course from scratch. The content presented below was designed with the assumption that you already have a preliminary understanding of Periodic Trends. Our goal is to help give you a more in-depth understanding of key outcomes, as well as to help you see how concepts relate/connect to one another within the scope of the course as a whole. We do our best to cover every topic within the unit; however, some testable outcomes may not be discussed. With that said, best of luck in your studying! Remember to make good use of your time, but to also take breaks as well. Yousef Abumustafa & Julia Ma The WebStraw McMaster Chemistry team WebStraw McMaster Periodic properties of elements The periodic table is divided into called columns called groups that group elements with similar chemical/physical properties together and rows called periods that group elements with the same -
![Atomic and Ionic Radii of Elements 1–96 Martinrahm,*[A] Roald Hoffmann,*[A] and N](https://docslib.b-cdn.net/cover/8398/atomic-and-ionic-radii-of-elements-1-96-martinrahm-a-roald-hoffmann-a-and-n-668398.webp)
Atomic and Ionic Radii of Elements 1–96 Martinrahm,*[A] Roald Hoffmann,*[A] and N
DOI:10.1002/chem.201602949 Full Paper & Elemental Radii Atomic and Ionic Radii of Elements 1–96 MartinRahm,*[a] Roald Hoffmann,*[a] and N. W. Ashcroft[b] Abstract: Atomic and cationic radii have been calculated for tive measureofthe sizes of non-interacting atoms, common- the first 96 elements, together with selected anionicradii. ly invoked in the rationalization of chemicalbonding, struc- The metric adopted is the average distance from the nucleus ture, and different properties. Remarkably,the atomic radii where the electron density falls to 0.001 electrons per bohr3, as defined in this way correlate well with van der Waals radii following earlier work by Boyd. Our radii are derived using derived from crystal structures. Arationalizationfor trends relativistic all-electron density functional theory calculations, and exceptionsinthose correlations is provided. close to the basis set limit. They offer asystematic quantita- Introduction cule,[2] but we prefer to follow through with aconsistent pic- ture, one of gauging the density in the atomic groundstate. What is the size of an atom or an ion?This question has been The attractivenessofdefining radii from the electron density anatural one to ask over the centurythat we have had good is that a) the electron density is, at least in principle, an experi- experimental metricinformation on atoms in every form of mental observable,and b) it is the electron density at the out- matter,and (more recently) reliable theory for thesesame ermost regionsofasystem that determines Pauli/exchange/ atoms. And the momentone asks this question one knows same-spinrepulsions, or attractive bondinginteractions, with that there is no unique answer.Anatom or ion coursing down achemical surrounding. -

ARC: an Open-Source Library for Calculating Properties of Alkali Rydberg Atoms
ARC: An open-source library for calculating properties of alkali Rydberg atoms N. Šibalic´a,∗, J. D. Pritchardb, C. S. Adamsa, K. J. Weatherilla aJoint Quantum Center (JQC) Durham-Newcastle, Department of Physics, Durham University, South Road, Durham, DH1 3LE, United Kingdom bDepartment of Physics, SUPA, University of Strathclyde, 107 Rottenrow East, Glasgow, G4 0NG, United Kingdom Abstract We present an object-oriented Python library for computation of properties of highly-excited Rydberg states of alkali atoms. These include single-body effects such as dipole matrix elements, excited-state lifetimes (radiative and black-body limited) and Stark maps of atoms in external electric fields, as well as two-atom interaction potentials accounting for dipole and quadrupole coupling effects valid at both long and short range for arbitrary placement of the atomic dipoles. The package is cross-referenced to precise measurements of atomic energy levels and features extensive documentation to facilitate rapid upgrade or expansion by users. This library has direct application in the field of quantum information and quantum optics which exploit the strong Rydberg dipolar interactions for two-qubit gates, robust atom-light interfaces and simulating quantum many-body physics, as well as the field of metrology using Rydberg atoms as precise microwave electrometers. Keywords: Alkali atom, Matrix elements, Dipole-dipole interactions, Stark shift, Förster resonances PROGRAM SUMMARY They are a flourishing field for quantum information process- Program Title: ARC: Alkali Rydberg Calculator ing [1, 2] and quantum optics [3, 4, 5] in the few to single exci- Licensing provisions: BSD-3-Clause tation regime, as well as many-body physics [6, 7, 8, 9, 10, 11], Programming language: Python 2.7 or 3.5, with C extension in the many-excitations limit. -

Periodic Trends and the S-Block Elements”, Chapter 21 from the Book Principles of General Chemistry (Index.Html) (V
This is “Periodic Trends and the s-Block Elements”, chapter 21 from the book Principles of General Chemistry (index.html) (v. 1.0M). This book is licensed under a Creative Commons by-nc-sa 3.0 (http://creativecommons.org/licenses/by-nc-sa/ 3.0/) license. See the license for more details, but that basically means you can share this book as long as you credit the author (but see below), don't make money from it, and do make it available to everyone else under the same terms. This content was accessible as of December 29, 2012, and it was downloaded then by Andy Schmitz (http://lardbucket.org) in an effort to preserve the availability of this book. Normally, the author and publisher would be credited here. However, the publisher has asked for the customary Creative Commons attribution to the original publisher, authors, title, and book URI to be removed. Additionally, per the publisher's request, their name has been removed in some passages. More information is available on this project's attribution page (http://2012books.lardbucket.org/attribution.html?utm_source=header). For more information on the source of this book, or why it is available for free, please see the project's home page (http://2012books.lardbucket.org/). You can browse or download additional books there. i Chapter 21 Periodic Trends and the s-Block Elements In previous chapters, we used the principles of chemical bonding, thermodynamics, and kinetics to provide a conceptual framework for understanding the chemistry of the elements. Beginning in Chapter 21 "Periodic Trends and the ", we use the periodic table to guide our discussion of the properties and reactions of the elements and the synthesis and uses of some of their commercially important compounds. -

Periodic Activity of Metals Periodic Trends and the Properties of the Elements SCIENTIFIC
Periodic Activity of Metals Periodic Trends and the Properties of the Elements SCIENTIFIC Introduction Elements are classified based on similarities, differences, and trends in their properties, including their chemical reactions. The reactions of alkali and alkaline earth metals with water are pretty spectacular chemical reactions. Mixtures bubble and boil, fizz and hiss, and may even smoke and burn. Introduce the study of the periodic table and periodic trends with this exciting demonstration of the activity of metals. Concepts • Alkali and alkaline earth metals • Periodic table and trends • Physical and chemical properties • Metal activity Materials Calcium turnings, Ca, 0.3 g Beaker, Berzelius (tall-form), Pyrex®, 500-mL, 4 Lithium metal, Li, precut piece Forceps or tongs Magnesium ribbon, Mg, 3-cm Knife (optional) Sodium metal, Na, precut piece Petri dishes, disposable, 4 Phenolphthalein, 1% solution, 2 mL Scissors Water, distilled or deionized, 600 mL Safety Precautions Lithium and sodium are flammable, water-reactive, corrosive solids; dangerous when exposed to heat or flame. They react violently with water to produce flammable hydrogen gas and solutions of corrosive metal hydroxides. Hydrogen gas may be released in sufficient quantities to cause ignition. Do NOT “scale up” this demonstration using larger pieces of sodium or lithium! These metals are shipped in dry mineral oil. Store them in mineral oil until immediately before use. Do not allow these metals to stand exposed to air from one class period to another or for extended periods of time. Purchasing small, pre-cut pieces of lithium and sodium greatly reduces their potential hazard. Calcium metal is flammable in finely divided form and reacts upon contact with water to give flammable hydrogen gas and corrosive calcium hydroxide. -

CHEMISTRY 130 Periodic Trends
CHEMISTRY 130 General Chemistry I Periodic Trends Elements within a period or group of the periodic table often show trends in physical and chemical properties. The variation in relative sizes of the halogens is shown above. DEPARTMENT OF CHEMISTRY UNIVERSITY OF KANSAS 1 Periodic Trends Introduction In the modern periodic table (shown below in Figure 1), elements are arranged according to increasing atomic number in horizontal rows called “periods.” In Figure 1, atomic numbers, which represent the number of protons in an atom of a given element, are listed directly above the element symbols. Figure 1: The modern periodic table. Elements in boxes shaded blue, orange, and purple are characterized as metals, metalloids, and nonmetals, respectively. The structure of the periodic table is such that elements with similar properties are aligned vertically in columns called “groups” or “families.” As indicated in Figure 1, each group has a number (1-18) associated with it. Select groups have also been assigned special names. For instance, the elements in Group 1 (hydrogen excluded) are called the alkali metals. These metallic elements react with oxygen to form bases. They also form alkaline (basic) solutions when mixed with water. Elements in other columns of the periodic table have also been given special “family” names. For instance, the elements in Groups 2, 11 (Cu, Ag, and Au), 16, 17, and 18 are commonly referred to as the alkaline earth metals, the coinage metals, the chalcogens, the halogens, and the noble gases, respectively. Many of these names reflect, unsurprisingly, the reactivity (or the lack thereof) of the elements found within the given group. -

Chalcogen-Nitrogen Bond: Insights Into a Key Chemical Motif
Proceedings Chalcogen-nitrogen Bond: Insights into A Key Chemical Motif Marco Bortoli,1 Andrea Madabeni,1 Pablo Andrei Nogara,2 Folorunsho B. Omage,2 Giovanni Ribaudo,3 Davide Zeppilli,1 Joao Batista Teixeira Rocha,2* Laura Orian1* 1 Dipartimento di Scienze Chimiche Università degli Studi di Padova Via Marzolo 1 35131 Padova, Italy; [email protected] (M.B.); [email protected] (A.M..); [email protected] (D.Z.) 2 Departamento de Bioquímica e Biologia Molecular, Universidade Federal de Santa Maria, Santa Maria, 97105-900, RS Brazil; [email protected] (P.A.N.); [email protected] (F.B.O.) 3 Dipartimento di Medicina Molecolare e Traslazionale, Università degli Studi di Brescia, Viale Europa 11, 25123 Brescia, Italy; [email protected] (G.R.) * Correspondence: : [email protected] (J.B.T.R), [email protected] (L.O.); † Presented at the 1st International Electronic Conference on Catalysis Sciences, 10–30 November 2020; Available online: https://eccs2020.sciforum.net/ Published: 10 November 2020 Abstract: Chalcogen-nitrogen chemistry deals with systems in which sulfur, selenium or tellurium is linked to a nitrogen nucleus. This chemical motif is a key component of different functional structures, ranging from inorganic materials and polymers to rationally designed catalysts, to bioinspired molecules and enzymes. The formation of a selenium-nitrogen bond, and its disruption, are rather common events in organic Se-catalyzed processes. In nature, along the mechanistic path of glutathione peroxidase, evidence of the formation of a Se-N bond in highly oxidizing conditions has been reported and interpreted as a strategy to protect the selenoenzyme from overoxidation. -

Periodic Trends
Periodic Trends There are three main properties of atoms that we are concerned with. Ionization Energy- the amount of energy need to remove an electron from an atom. Atomic Radius- the estimate of atomic size based on covalent bonding. Electron Affinity- the amount of energy absorbed or released when an atom gains an electron. All three properties have regularly varying trends we can identify in the periodic table and all three properties can be explained using the same properties of electronic structure. Explaining Periodic Trends All of the atomic properties that we are interested in are caused by the attractive force between the positively charged nucleolus and the negatively charged electrons. We need to consider what would affect that relationship. The first thing to consider is the effective nuclear charge. We need to look at the number of protons. Secondly we will need to look at the nucleolus - electron distance and the electron shielding. Lastly we will need to consider electron - electron repulsion. Ionization Energy As we move across a period the general trend is an increase in IE. However when we jump to a new energy level the distance increases and the amount of e-- shielding increases dramatically. Reducing the effective nuclear charge. In addition to the over all trend we need to explain the discrepancies. The discrepancies can be explained by either electron shielding or by electron electron repulsion. 1 Atomic Radius The trend for atomic radius is to decrease across a period and to increase down a family. These trends can be explained with the same reasons as IE. -

Periodic Trends Remember from the "Periodic Table" Notes
November 07, 2014 Periodic Trends Remember from the "Periodic Table" Notes... • The periodic table is a tabular display of the chemical elements, organized by their atomic number, electron configuration, and recurring properties. • Periodic law: There is a periodic repetition of chemical and physical properties of the elements when they are arranged by increasing atomic number November 07, 2014 Atomic Radius Graph • What are some initial observations about the atomic radius data/graph? • What is atomic radius? November 07, 2014 Go finish the rest of the worksheet with your group! You have 20 minutes. November 07, 2014 Atomic Radius Trend Discussion • What happens to atomic radius as you go across the period? Why? • What happens to atomic radius as you go down the group? Why? November 07, 2014 Periodic Trends Notes Get your handout out! November 07, 2014 Why is it called a periodic table? • The properties of the elements in the table repeat in a "periodic" way (specific pattern). • Periodic law: There is a periodic repetition of chemical and physical properties of the elements when they are arranged by increasing atomic number • The modern periodic table is arranged by > atomic number = # of protons > properties > electron configuration November 07, 2014 Periodic Law • Now lets look at some properties of elements > We looked at some of these in "Meet My Family"! Alkali Metals Halogens November 07, 2014 Periodic Trends • Chemical properties of elements are determined by their electron configuration. • Properties are periodic because the number of valence electrons is periodic. November 07, 2014 Electron Configuration and the Periodic Table • Remember electrons are found in atomic orbitals > Principle energy level (n, shells) tells us the relative size and energy of atomic orbitals.