(Sistrurus Catenatus Edwardsii) and Western Massasauga
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Gorilla in a Tutu Principle Or, Pecan Pie at Minnie and Earl's
The Gorilla in a Tutu Principle or, Pecan Pie at Minnie and Earl’s Adam-Troy Castro Prior Analog stories set against the backdrop of this very strange period in the history of lunar colonization were "Sunday Night Yams at Minnie and Earl's" (June 2001) and "Gunfight at Farside" (April 2009). Many years ago—and when a man as old as I am uses the phrase, “many years ago,” he means a lifetime—I told Minnie, “I’m an engineer, not a poet.” Minnie was a dear old gal of unfailing honesty, with a central role in what follows. I was in love with her eyes. I don’t mean this in a sexual way. The difference between our ages, and certainly our backgrounds, would have made that grotesque. But her eyes were rich and deep, and filled with an understanding of life’s greatest mysteries, that made them a perfect place to lose yourself when she was pointing out how silly you are. I haven’t seen her or her husband Earl in decades, but I can picture those eyes like it was yesterday. When I told her I wasn’t a poet, she said, “How dare you. It’s okay to operate under a poetry deficit, but to imply that deficit for an entire profession is dishonest. I’ve known more than my share of engineers, and any number of poets among them. Great engineering is poetry.” I suppose she was right. She was, in most things. Regardless, I can speak for myself. I’m not a poet, not even in the sense that sweet lady meant. -
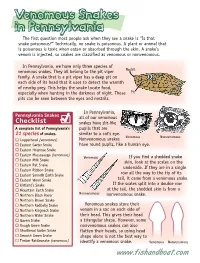
Venomous Snakes in Pennsylvania the First Question Most People Ask When They See a Snake Is “Is That Snake Poisonous?” Technically, No Snake Is Poisonous
Venomous Snakes in Pennsylvania The first question most people ask when they see a snake is “Is that snake poisonous?” Technically, no snake is poisonous. A plant or animal that is poisonous is toxic when eaten or absorbed through the skin. A snake’s venom is injected, so snakes are classified as venomous or nonvenomous. In Pennsylvania, we have only three species of venomous snakes. They all belong to the pit viper Nostril family. A snake that is a pit viper has a deep pit on each side of its head that it uses to detect the warmth of nearby prey. This helps the snake locate food, especially when hunting in the darkness of night. These Pit pits can be seen between the eyes and nostrils. In Pennsylvania, Pennsylvania Snakes all of our venomous Checklist snakes have slit-like A complete list of Pennsylvania’s pupils that are 21 species of snakes. similar to a cat’s eye. Venomous Nonvenomous Copperhead (venomous) Nonvenomous snakes Eastern Garter Snake have round pupils, like a human eye. Eastern Hognose Snake Eastern Massasauga (venomous) Venomous If you find a shedded snake Eastern Milk Snake skin, look at the scales on the Eastern Rat Snake underside. If they are in a single Eastern Ribbon Snake Eastern Smooth Earth Snake row all the way to the tip of its Eastern Worm Snake tail, it came from a venomous snake. Kirtland’s Snake If the scales split into a double row Mountain Earth Snake at the tail, the shedded skin is from a Northern Black Racer Nonvenomous nonvenomous snake. -

Cottonmouth Snake Bites Reported to the Toxic North American Snakebite Registry 2013–2017
Clinical Toxicology ISSN: 1556-3650 (Print) 1556-9519 (Online) Journal homepage: https://www.tandfonline.com/loi/ictx20 Cottonmouth snake bites reported to the ToxIC North American snakebite registry 2013–2017 K. Domanski, K. C. Kleinschmidt, S. Greene, A. M. Ruha, V. Berbata, N. Onisko, S. Campleman, J. Brent, P. Wax & on behalf of the ToxIC North American Snakebite Registry Group To cite this article: K. Domanski, K. C. Kleinschmidt, S. Greene, A. M. Ruha, V. Berbata, N. Onisko, S. Campleman, J. Brent, P. Wax & on behalf of the ToxIC North American Snakebite Registry Group (2019): Cottonmouth snake bites reported to the ToxIC North American snakebite registry 2013–2017, Clinical Toxicology, DOI: 10.1080/15563650.2019.1627367 To link to this article: https://doi.org/10.1080/15563650.2019.1627367 Published online: 13 Jun 2019. Submit your article to this journal Article views: 38 View Crossmark data Full Terms & Conditions of access and use can be found at https://www.tandfonline.com/action/journalInformation?journalCode=ictx20 CLINICAL TOXICOLOGY https://doi.org/10.1080/15563650.2019.1627367 CLINICAL RESEARCH Cottonmouth snake bites reported to the ToxIC North American snakebite registry 2013–2017 K. Domanskia, K. C. Kleinschmidtb, S. Greenec , A. M. Ruhad, V. Berbatae, N. Oniskob, S. Camplemanf, J. Brente, P. Waxb and on behalf of the ToxIC North American Snakebite Registry Group aReno School of Medicine, University of Nevada, Reno, NV, USA; bSouthwestern Medical Center, University of Texas, Dallas, TX, USA; cBaylor College of Medicine, Houston, TX, USA; dBanner Good Samaritan Medical Center, Phoenix, AZ, USA; eEmergency Medicine, Medical Toxicology, University of Colorado, Denver, CO, USA; fAmerican College of Medical Toxicology, Phoenix, AZ, USA ABSTRACT ARTICLE HISTORY Introduction: The majority of venomous snake exposures in the United States are due to snakes from Received 9 April 2019 the subfamily Crotalinae (pit vipers). -

Abstracts and Backgrounds
Abstracts and Backgrounds NAVY Con TABLE OF CONTENTS DESTINATION UNKNOWN ................................................................................. 3 WAR AND SOCIETY ............................................................................................. 5 MATT BUCHER – POTEMKIN PARADISE: THE UNITED FEDERATION IN THE 24TH CENTURY ............ 5 ELSA B. KANIA – BEYOND LOYALTY, DUTY, HONOR: COMPETING PARADIGMS OF PROFESSIONALISM IN THE CIVIL-MILITARY RELATIONS OF BABYLON 5 ............................................ 6 S.H. HARRISON – STAR CULTURE WARS: THE NEGATIVE IMPACT OF POLITICS AND IMPERIALISM ON IMPERIAL NAVAL CAPABILITY IN STAR WARS ................................................................................ 6 MATTHEW ADER – THE ARISTOCRATS STRIKE BACK: RE-ECALUATING THE POLITICAL COMPOSITION OF THE ALLIANCE TO RESTORE THE REPUBLIC ......................................................... 7 LT COL BREE FRAM, USSF – LEADERSHIP IN TRANSITION: LESSONS FROM TRILL .......................... 7 PAST AND FUTURE COMPETITION ................................................................ 8 WILLIAM J. PROM – THE ONCE AND FUTURE KING OF BATTLE: ARTILLERY (AND ITS ABSENCE) IN SCIENCE FICTION .......................................................................................................................... 8 TOM SHUGART – ALL ABOUT EVE: WHAT VIRTUAL FOREVER WARS CAN TEACH US ABOUT THE FUTURE OF COMBAT ................................................................................................................... 10 -

Ecology of the Desert Massasauga Rattlesnake in Colorado: Habitat and Resource Utilization
Ecology of the Desert Massasauga Rattlesnake in Colorado: Habitat and Resource Utilization A report to the CDOW and USFWS for the Colorado Wildlife Conservation Grant Program Dr. Stephen P. Mackessy School of Biological Sciences University of Northern Colorado 501 20th St., CB 92 Greeley, CO 80639-0017 USA Tel: (970)-351-2429 Fax: (970)-351-2335 Email: [email protected] http://www.unco.edu/nhs/biology/faculty_staff/mackessy_stephen.htm Desert Massasauga (Sistrurus catenatus edwardsii) from Lincoln County, Colorado ABSTRACT We conducted a radiotelemetric study on a population of Desert Massasaugas (Sistrurus catenatus edwardsii) in Lincoln County, SE Colorado. Massasaugas were most active between 14 and 30 °C, with an average ambient temperature during activity of 22.1°C. In the spring, snakes make long distance movements (up to 2 km) from the hibernaculum (shortgrass, compacted clay soils) to summer foraging areas (mixed grass/sandsage, sand hills). Summer activity is characterized by short distance movements, and snakes are most often observed at the base of sandsage in ambush or resting coils. Massasaugas gave birth to 5-7 young in late August, and reproduction appears to be biennial. Observations on three radioed gravid females indicated that Desert Massasaugas show maternal attendance for at least five days post-parturition. Snakes returned to the hibernaculum area in October and appear to hibernate individually in rodent burrows most commonly. However, the immediate region is utilized by several other species of snakes, particularly Prairie Rattlesnakes (Crotalus viridis viridis), and Massasaugas were also observed at the entrance to these den sites, suggesting that they are used by the Massasaugas as well. -

The Eastern Massasauga Rattlesnake Is Toxic, but Only a Small Amount Is Injected Through Short Fangs
What’s so special about the massasauga? Northern Indiana’s The rare eastern massasauga is northern Indiana’s only native rattlesnake. Endangered Because eastern massasaugas are shy and secretive, you may never see one in the wild. These snakes hide under Rattlesnake brush and retreat to a sheltered area if spotted in the open. Although eastern massasaugas are venomous, they usually flee from humans rather than bite. Their venom Range of the Eastern Massasauga Rattlesnake is toxic, but only a small amount is injected through short fangs. The last human fatality from an eastern Why are they endangered? massasauga bite occurred more than 60 years ago. Eastern massasaugas are endangered over much of their Eastern massasaugas live in range because their wetland habitats are often drained and northern Indiana’s swamps, Eastern massasaugas play an important role in the filled for development. These snakes are also collected for bogs, and wetlands. ecosystem by feeding on mice, voles, and shrews, thus the illegal reptile trade. People may kill massasaugas out of Christopher Smith helping to keep the rodent population under control. fear, not realizing the snakes’ importance to the ecosystem. People and snakes can live together. Nicholas Scobel Understanding snakes is the first step. Learn to identify eastern massasaugas and other Indiana snakes. A field guide to native reptiles can help. Wear proper footwear when hiking in areas where snakes might be found, especially at night. Stay on trails. Limit the use of pesticides and other chemicals on natural areas on your property. All wildlife will benefit. Teach your family and friends about snakes and what to do if they see an eastern massasauga. -

Jennifer Szymanski Usfish and Wildlife Service Endangered
Written by: Jennifer Szymanski U.S.Fish and Wildlife Service Endangered Species Division 1 Federal Drive Fort Snelling, Minnesota 55111 Acknowledgements: Numerous State and Federal agency personnel and interested individuals provided information regarding Sistrurus c. catenatus’status. The following individuals graciously provided critical input and numerous reviews on portions of the manuscript: Richard Seigel, Robert Hay, Richard King, Bruce Kingsbury, Glen Johnson, John Legge, Michael Oldham, Kent Prior, Mary Rabe, Andy Shiels, Doug Wynn, and Jeff Davis. Mary Mitchell and Kim Mitchell provided graphic assistance. Cover photo provided by Bruce Kingsbury Table of Contents Taxonomy....................................................................................................................... 1 Physical Description....................................................................................................... 3 Distribution & State Status............................................................................................. 3 Illinois................................................................................................................. 5 Indiana................................................................................................................ 5 Iowa.................................................................................................................... 5 Michigan............................................................................................................ 6 Minnesota.......................................................................................................... -

The Massasauga Rattlesnake
Pennsylvania Amphibians & Reptiles TTheh e MMassasauga s s a s a u g a R a t t l e s n a k e by Andrew L. Shiels “Massasauga,” “swamp rattler,” and “black snapper,” are a 24 to 30 inches. It has a grayish body color with a series of few of the nicknames given to a rare, secretive, yet interest- dark-brown, irregularly shaped blotches along the top of ing rattlesnake found in northwestern Pennsylvania. Its the back. These blotches are the source of the Latin spe- limited distribution and unique ecological needs have un- cies name “catenatus,” which means “chain-like.” Along fortunately placed this species among Pennsylvania’s most the sides of the body are two to three rows of smaller, more endangered. Out of sight and out of mind to most Penn- rounded spots. sylvanians, the massasauga has been quietly disappearing Massasaugas are pit vipers, which means there is a for years. However, recent conservation efforts have been heat-sensing pit on the side of the head between the eye undertaken to ensure its survival in the Commonwealth. and nostril. Like our other two venomous species, the Understanding its biology and ecology are keys to devel- northern copperhead and the eastern timber rattlesnake, oping an effective conservation strategy. massasaugas have vertically elliptical pupils and a single The eastern massasauga (Sistrurus catenatus catenatus) row of scales on the underside of the tail. Unlike timber is a medium-sized venomous snake that reaches lengths of rattlesnakes, which have many small scales on the head, a massasauga’s head is covered with nine large, scale plates typical of our non-venomous species. -

The Indigenous As Alien
Volpp_production read v5 (clean) (Do Not Delete) 6/28/2015 9:21 PM The Indigenous As Alien Leti Volpp* I. Space, Time, Membership ......................................................................................... 293 II. Indians As Aliens and Citizens ................................................................................ 300 III. The Political Theory of Forgetting—The Settler’s Alibi .................................. 316 Immigration law, as it is taught, studied, and researched in the United States, imagines away the fact of preexisting indigenous peoples. Why is this the case? I argue, first, that this elision reflects and reproduces how the field of immigration law narrates space, time, and national membership. But despite their disappearance from the field, Indians have figured in immigration law, and thus I describe the neglected legal history of the treatment of Indians under U.S. immigration and citizenship law.1 The Article then returns to explain why indigenous people have disappeared from immigration law through an investigation of the relationship between “We the People,” the “settler contract,” and the “nation of immigrants.” The story of the field of U.S. immigration law is typically a narrative of the assertion of national sovereign power that begins in the late 1880s with a trilogy of * Robert D. and Leslie Kay Raven Professor of Law, University of California, Berkeley School of Law, [email protected]. Many thanks to the UCLA Critical Race Studies Symposium on Race and Sovereignty, where I first began -

Lesser Known Snakes (PDF)
To help locate food, snakes repeat- edly flick out their narrow forked tongues to bring odors to a special sense organ in their mouths. Snakes are carnivores, eating a variety of items, including worms, with long flexible bodies, snakes insects, frogs, salamanders, fish, are unique in appearance and small birds and mammals, and even easily recognized by everyone. other snakes. Prey is swallowed Unfortunately, many people are whole. Many people value snakes afraid of snakes. But snakes are for their ability to kill rodent and fascinating and beautiful creatures. insect pests. Although a few snakes Their ability to move quickly and are venomous, most snakes are quietly across land and water by completely harmless to humans. undulating their scale-covered Most of the snakes described bodies is impressive to watch. here are seldom encountered— Rather than moveable eyelids, some because they are rare, but snakes have clear scales covering most because they are very secre- their eyes, which means their eyes tive, usually hiding under rocks, are always open. Some snake logs or vegetation. For information species have smooth scales, while on the rest of New York’s 17 species others have a ridge on each scale of snakes, see the August 2001 that gives it a file-like texture. issue of Conservationist. Text by Alvin Breisch and Richard C. Bothner, Maps by John W. Ozard (based on NY Amphibian & Reptile Atlas Project) Artwork by Jean Gawalt, Design by Frank Herec This project was funded in part by Return A Gift To Wildlife tax check-off, U.S. Fish & Wildlife Service and the NYS Biodiversity Research Institute. -

The Venomous Snakes of Texas Health Service Region 6/5S
The Venomous Snakes of Texas Health Service Region 6/5S: A Reference to Snake Identification, Field Safety, Basic Safe Capture and Handling Methods and First Aid Measures for Reptile Envenomation Edward J. Wozniak DVM, PhD, William M. Niederhofer ACO & John Wisser MS. Texas A&M University Health Science Center, Institute for Biosciences and Technology, Program for Animal Resources, 2121 W Holcombe Blvd, Houston, TX 77030 (Wozniak) City Of Pearland Animal Control, 2002 Old Alvin Rd. Pearland, Texas 77581 (Niederhofer) 464 County Road 949 E Alvin, Texas 77511 (Wisser) Corresponding Author: Edward J. Wozniak DVM, PhD, Texas A&M University Health Science Center, Institute for Biosciences and Technology, Program for Animal Resources, 2121 W Holcombe Blvd, Houston, TX 77030 [email protected] ABSTRACT: Each year numerous emergency response personnel including animal control officers, police officers, wildlife rehabilitators, public health officers and others either respond to calls involving venomous snakes or are forced to venture into the haunts of these animals in the scope of their regular duties. North America is home to two distinct families of native venomous snakes: Viperidae (rattlesnakes, copperheads and cottonmouths) and Elapidae (coral snakes) and southeastern Texas has indigenous species representing both groups. While some of these snakes are easily identified, some are not and many rank amongst the most feared and misunderstood animals on earth. This article specifically addresses all of the native species of venomous snakes that inhabit Health Service Region 6/5s and is intended to serve as a reference to snake identification, field safety, basic safe capture and handling methods and the currently recommended first aide measures for reptile envenomation. -

D:\Downloads\Animorphs\Docs\K. A. Applegate
1 Animorphs Volume 08 The Alien K.A. Applegate *Converted to EBook by Dace K 2 Prologue Before Earth . <Prepare for return to normal space,> Captain Nerefir said in thought-speak. I was on the bridge of our Dome ship. It was an amazing moment. I had never been on the bridge before. I'd always been stuck in my quarters, or up in the dome. It was an honor to be on the battle bridge with the full warriors, the princes, and the captain himself. It was because I was Elfangor's little brother. An aristh like me, a warrior-cadet, wouldn't have been on the bridge otherwise. Especially not an aristh who had once run into Captain Nerefir so hard he'd fallen over and ended up bruising one of his eye stalks. It was an accident, but still, it's just not a good idea for lowly cadets to go plowing into great heroes. But everyone loved Elfangor, so they had to tolerate me. That's the story of my life. If I live two hundred years, I'll probably still be known as Elfangor's little brother. We came out of Z-space or Zero-space, a realm of white emptiness, back into normal space. Through the monitors I saw nothing but blackness dotted with stars. And there, just ahead of us, no more than a half-million miles away, was a small, mostly blue planet. <Is that Earth?> I asked Elfangor. <I didn't realize there was so much water. Can you get Old Hoof and Tail to let me go down to the planet with you?> <Aximili, shut up!> Elfangor said quickly.