Proquest Dissertations
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pollination of Two Species of Ferocactus: Interactions Between Cactus-Specialist Bees and Their Host Plants
Functional Blackwell Publishing, Ltd. Ecology 2005 Pollination of two species of Ferocactus: interactions 19, 727–734 between cactus-specialist bees and their host plants M. E. MCINTOSH Department of Ecology and Evolutionary Biology, 1041 E. Lowell Street; BioSciences West, Room 310, University of Arizona, Tucson, AZ 85721, USA Summary 1. Resolving the controversy over the prevalence of generalization in plant–pollinator interactions requires field studies characterizing the pollination effectiveness of all a plant’s floral visitors. Herein, the pollination effectiveness of all visitors to two species of barrel cactus (Ferocactus) was quantified. 2. Flowers of both species were pollinated almost exclusively by cactus-specialist bees: 99% (F. cylindraceus (Engelm.) Orcutt) and 94% (F. wislizeni (Engelm.) Britt. and Rose) of all seeds produced in this study resulted from cactus bee visits. 3. For F. cylindraceus, the cactus-specialist Diadasia rinconis was the most abundant visitor. For F. wislizeni, three cactus-specialists (including D. rinconis) plus generalists in the family Halictidae (which did not act as pollinators) each accounted for a quarter of all visits. 4. Diadasia rinconis visits to F. wislizeni flowers were more effective (per-visit) than visits by the other two cactus-specialists. 5. Pollen-collecting and nectar-collecting visits were equally effective. Nectar-collecting visits were the most abundant. 6. Apart from the non-pollinating halictids, floral visitors surprisingly did not include commonly co-occurring generalist bees. 7. These data suggest that, just as apparently specialized flowers may be visited by a diverse assemblage of generalists, so apparently generalized flowers may be visited predominantly by specialists, and that these specialists may perform virtually all of the pollination. -

Relationship of Insects to the Spread of Azalea Flower Spot
TECHNICAL BULLETIN NO. 798 • JANUARY 1942 Relationship of Insects to the Spread of Azalea Flower Spot By FLOYD F. SMITH Entomologist» Division of Truck Crop and Garden Insect Investigations Bureau of Entomology and Plant Quarantine and FREJEMAN WEISS Senior Pathologist, Division of Mycology and Disease Survey Bureau of Plant Industry UNITED STATES DEPARTMENT OF AGRICULTURE, WASHINGTON* D* C. For sale by the Superintendent of'Documents, Washington, D. G. • Price 10 cents Technical Bulletin No. 798 • January 1942 Relationship of Insects to the Spread of Azalea Flower Spot ^ By FLOYD F. SMITH, entomologist, Division of Truck Crop and Garden Insect Investigations, Bureau of Entomology and Plant Quarantine, and FREEMAN WEISS, senior pathologist. Division of Mycology and Disease Survey, Bureau of Plant Industry ^ CONTENTS Page Page Introduction ' 1 Disease transmission by insects II Insects visiting azaleas and observations on Preliminary studies, 1934 and 1935 11 their habits 2 Improved methods for collecting insects Bumblebees 2 and testing their infectivity 12 Carpenter bees 4 Studies in 1936 18 Ground-nesting bees 5 Transmission of flower spot on heads or Honeybees 5 legs or on pollen from insects- 20 Thrips 5 Transmission tests in 1937 and 1938 20 Ants 5 Relationship of insects to primary infection. 29 Flies 6 Other relationships of insects to the disease 33 Activity of bees in visiting flowers 6 Control experiments with insects on azaleas -. 39 Cause of insect abrasions and their relationship * E fîect of insecticid al dusts on bees 39 to flower spot infection < 7 Eiïect of poisoned sprays on bees 40 Occurrence on insects of conidia of the organ- Discussion of results 40 ism causing azalea flower spot 10 Summary 41 INTRODUCTION A serious spot disease and tlight was first reported in April 1931 near Charleston, S. -

Universidad Pol Facultad D Trabajo
UNIVERSIDAD POLITÉCNICA DE MADRID FACULTAD DE INFORMÁTICA TRABAJO FINAL DE CARRERA ESTUDIO DEL PROTOCOLO XMPP DE MESAJERÍA ISTATÁEA, DE SUS ATECEDETES, Y DE SUS APLICACIOES CIVILES Y MILITARES Autor: José Carlos Díaz García Tutor: Rafael Martínez Olalla Madrid, Septiembre de 2008 2 A mis padres, Francisco y Pilar, que me empujaron siempre a terminar esta licenciatura y que tanto me han enseñado sobre la vida A mis abuelos (q.e.p.d.) A mi hijo icolás, que me ha dejado terminar este trabajo a pesar de robarle su tiempo de juego conmigo Y muy en especial, a Susana, mi fiel y leal compañera, y la luz que ilumina mi camino Agradecimientos En primer lugar, me gustaría agradecer a toda mi familia la comprensión y confianza que me han dado, una vez más, para poder concluir definitivamente esta etapa de mi vida. Sin su apoyo, no lo hubiera hecho. En segundo lugar, quiero agradecer a mis amigos Rafa y Carmen, su interés e insistencia para que llegara este momento. Por sus consejos y por su amistad, les debo mi gratitud. Por otra parte, quiero agradecer a mis compañeros asesores militares de Nextel Engineering sus explicaciones y sabios consejos, que sin duda han sido muy oportunos para escribir el capítulo cuarto de este trabajo. Del mismo modo, agradecer a Pepe Hevia, arquitecto de software de Alhambra Eidos, los buenos ratos compartidos alrrededor de nuestros viejos proyectos sobre XMPP y que encendieron prodigiosamente la mecha de este proyecto. A Jaime y a Bernardo, del Ministerio de Defensa, por haberme hecho descubrir las bondades de XMPP. -

On the Pima County Multi-Species Conservation Plan, Arizona
United States Department of the Interior Fish and ,Vildlife Service Arizona Ecological Services Office 2321 West Royal Palm Road, Suite 103 Phoenix, Arizona 85021-4951 Telephone: (602) 242-0210 Fax: (602) 242-2513 In reply refer to: AESO/SE 22410-2006-F-0459 April 13, 2016 Memorandum To: Regional Director, Fish and Wildlife Service, Albuquerque, New Mexico (ARD-ES) (Attn: Michelle Shaughnessy) Chief, Arizona Branch, Re.. gul 7/to . D'vision, Army Corps of Engineers, Phoenix, Arizona From: Acting Field Supervisor~ Subject: Biological and Conference Opinion on the Pima County Multi-Species Conservation Plan, Arizona This biological and conference opinion (BCO) responds to the Fish and Wildlife Service (FWS) requirement for intra-Service consultation on the proposed issuance of a section lO(a)(l)(B) incidental take permit (TE-84356A-O) to Pima County and Pima County Regional Flood Control District (both herein referenced as Pima County), pursuant to section 7 of the Endangered Species Act of 1973 (U.S.C. 1531-1544), as amended (ESA), authorizing the incidental take of 44 species (4 plants, 7 mammals, 8 birds, 5 fishes, 2 amphibians, 6 reptiles, and 12 invertebrates). Along with the permit application, Pima County submitted a draft Pima County Multi-Species Conservation Plan (MSCP). On June 10, 2015, the U.S. Army Corps of Engineers (ACOE) requested programmatic section 7 consultation for actions under section 404 of the Clean Water Act (CW A), including two Regional General Permits and 16 Nationwide Permits, that are also covered activities in the MSCP. This is an action under section 7 of the ESA that is separate from the section 10 permit issuance to Pima Couny. -
Download Windows Live Messenger for Linux Ubuntu
Download windows live messenger for linux ubuntu But installing applications in Ubuntu that were originally made for I found emescene to be the best Msn Messenger for Ubuntu Linux so far. It really gives you the feel as if you are using Windows Live Messenger. Its builds are available for Archlinux, Debian, Ubuntu, Fedora, Mandriva and Windows. At first I found it quite difficult to use Pidgin Internet Messenger on Ubuntu Linux. Even though it allows signing into MSN, Yahoo! Messenger and Google Talk. While finding MSN Messenger for Linux / Ubuntu, I found different emesene is also available and could be downloaded and installed for. At first I found it quite difficult to use Pidgin Internet Messenger on Ubuntu Linux. Even though it allows signing into MSN, Yahoo! Messenger. A simple & beautiful app for Facebook Messenger. OS X, Windows & Linux By downloading Messenger for Desktop, you acknowledge that it is not an. An alternative MSN Messenger chat client for Linux. It allows Linux users to chat with friends who use MSN Messenger in Windows or Mac OS. The strength of. Windows Live Messenger is an instant messenger application that For more information on installing applications, see InstallingSoftware. sudo apt-get install chromium-browser. 2. After the installation is Windows Live Messenger running in LinuxMint / Ubuntu. You can close the. Linux / X LAN Messenger for Debian/Ubuntu LAN Messenger for Fedora/openSUSE Download LAN Messenger for Windows. Windows installer A MSN Messenger / Live Messenger client for Linux, aiming at integration with the KDE desktop Ubuntu: Ubuntu has KMess in its default repositories. -

Crab Spiders Impact Floral-Signal Evolution Indirectly Through Removal
ARTICLE DOI: 10.1038/s41467-018-03792-x OPEN Crab spiders impact floral-signal evolution indirectly through removal of florivores Anina C. Knauer1, Moe Bakhtiari1,2 & Florian P. Schiestl1 The puzzling diversity of flowers is primarily shaped by selection and evolutionary change caused by the plant’s interaction with animals. The contribution of individual animal species to net selection, however, may vary depending on the network of interacting organisms. Here 1234567890():,; we document that in the buckler mustard, Biscutella laevigata, the crab spider Thomisus onustus reduces bee visits to flowers but also benefits plants by feeding on florivores. Uninfested plants experience a trade-off between pollinator and spider attraction as both bees and crab spiders are attracted by the floral volatile β-ocimene. This trade-off is reduced by the induced emission of β-ocimene after florivore infestation, which is stronger in plant populations where crab spiders are present than where they are absent, suggesting that plants are locally adapted to the presence of crab spiders. Our study demonstrates the context-dependence of selection and shows how crab spiders impact on floral evolution. 1 Department of Systematic and Evolutionary Botany, University of Zurich, Zollikerstrasse 107, 8008 Zurich, Switzerland. 2Present address: Institute of Biology, University of Neuchatel, Rue Emile-Argand 11, 2000 Neuchatel, Switzerland. Correspondence and requests for materials should be addressedto F.P.S. (email: fl[email protected]) NATURE COMMUNICATIONS | (2018) 9:1367 | DOI: 10.1038/s41467-018-03792-x | www.nature.com/naturecommunications 1 ARTICLE NATURE COMMUNICATIONS | DOI: 10.1038/s41467-018-03792-x lant–animal interactions are a major driver of plant Crab spiders camouflage themselves on flowers to hunt flower- evolution, including both local adaptation and species visiting insects such as pollinators (Fig. -

Apoidea: Andrenidae: Panurginae)
Contemporary distributions of Panurginus species and subspecies in Europe (Apoidea: Andrenidae: Panurginae) Sébastien Patiny Abstract The largest number of Old World Panurginus Nylander, 1848 species is distributed in the West- Palaearctic. The genus is absent in Africa and rather rare in the East-Palaearctic. Warncke (1972, 1987), who is the main author treating Palaearctic Panurginae in the last decades, subdivided Panurginus into a small number of species, including two principal taxa admitting for each a very large number of subspecies: Panurginus brullei (Lepeletier, 1841) and Panurginus montanus Giraud, 1861. Following recent works, the two latter are in fact complexes of closely related species. In the West-Palaearctic context, distributions of certain species implied in these complexes appear as very singular, distinct of mostly all other Panurginae ranges and highly interesting from a fundamental point of view. Based on a cartographic approach, the causes which influence (or have conditioned, in the past) the observed ranges of these species are discussed. Key words: Panurginae, biogeography, West-Palaearctic, speciation, glaciation. Introduction 1841) sensu lato are studied in the present paper. In the Old World Panurginae fauna, Panurginus The numerous particularities of their distribu- Nylander, 1848 is one of the only two genera tions are characterized and discussed here. The (with Melitturga Latreille, 1809) which are limits of these distributions were proposed and distributed in the entire Palaearctic region. The related to the contemporary and past develop- genera Camptopoeum Spinola, 1843 and Panur- ments which could have caused these ranges. gus Panzer, 1806 are also represented in the East- Palaearctic (in northern Thailand), but too few Catalogue of the old world species data are available for these genera to make them of Panurginus the subject of particular considerations. -
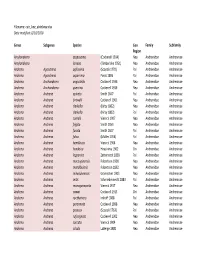
Anthophila List
Filename: cuic_bee_database. -

1. Padil Species Factsheet Scientific Name: Common Name Image
1. PaDIL Species Factsheet Scientific Name: Panurginus ineptus Cockerell, 1922 (Hymenoptera: Andrenidae: Panurginae: Panurgini) Common Name Tribe Representative - Panurgini Live link: http://www.padil.gov.au/pollinators/Pest/Main/139784 Image Library Australian Pollinators Live link: http://www.padil.gov.au/pollinators/ Partners for Australian Pollinators image library Western Australian Museum https://museum.wa.gov.au/ South Australian Museum https://www.samuseum.sa.gov.au/ Australian Museum https://australian.museum/ Museums Victoria https://museumsvictoria.com.au/ 2. Species Information 2.1. Details Specimen Contact: Museum Victoria - [email protected] Author: Ken Walker Citation: Ken Walker (2010) Tribe Representative - Panurgini(Panurginus ineptus)Updated on 8/11/2010 Available online: PaDIL - http://www.padil.gov.au Image Use: Free for use under the Creative Commons Attribution-NonCommercial 4.0 International (CC BY- NC 4.0) 2.2. URL Live link: http://www.padil.gov.au/pollinators/Pest/Main/139784 2.3. Facets Bio-Region: USA and Canada, Europe and Northern Asia Host Family: Not recorded Host Genera: Fresh Flowers Status: Exotic Species not in Australia Bio-Regions: Palaearctic, Nearctic Body Hair and Scopal location: Body hair relatively sparse, Tibia Episternal groove: Present but not extending below scrobal groove Wings: Submarginal cells - Three, Apex of marginal cell truncate or rounded Head - Structures: Two subantennal sutures below each antennal socket, Facial fovea present usually as a broad groove, Facial -

Initial Study / Environmental Assessment Annotated
This page has been intentionally left blank. This page has been intentionally left blank. SCH: PROPOSED MITIGATED NEGATIVE DECLARATION Pursuant to: Division 13, Public Resources Code Project Description The Los Angeles County Metropolitan Transportation Authority (Metro), in cooperation with the Gateway Cities Council of Governments and the California Department of Transportation (Caltrans) District 7, proposes to develop and implement an auxiliary lane on Eastbound State Route 91 within a 1.4-mile segment from the southbound Interstate 710 (I-710) interchange connector to eastbound State Route 91, to Cherry Avenue. The Study Area includes Eastbound State Route 91 (Post Miles [PM] R11.8/R13.2) and is located in the City of Long Beach and adjacent to the City of Paramount, California. Determination This proposed Mitigated Negative Declaration is included to give notice to interested agencies and the public that it is Caltrans’ intent to adopt a Mitigated Negative Declaration for this project. This does not mean that Caltrans’ decision regarding the Project is final. This Mitigated Negative Declaration is subject to change based on comments received by interested agencies and the public. All Project features (including standard practices and specifications) are considered in significance determinations. Caltrans has prepared an Initial Study for this project and, pending public review, expects to determine from this study that the proposed Project would not have a significant effect on the environment for the following reasons: The Project would have no effect on aesthetics, agriculture and forest resources, cultural resources, energy, land use and planning, mineral resources, population and housing, recreation, tribal cultural resources, and wildfire. -

Components of Nest Provisioning Behavior in Solitary Bees (Hymenoptera: Apoidea)*
Apidologie 39 (2008) 30–45 Available online at: c INRA/DIB-AGIB/ EDP Sciences, 2008 www.apidologie.org DOI: 10.1051/apido:2007055 Review article Components of nest provisioning behavior in solitary bees (Hymenoptera: Apoidea)* John L. Neff Central Texas Melittological Institute, 7307 Running Rope, Austin, Texas 78731, USA Received 13 June 2007 – Revised 28 September 2007 – Accepted 1 October 2007 Abstract – The components of nest provisioning behavior (resources per cell, transport capacity, trip dura- tion, trips per cell) are examined for a data set derived from the literature and various unpublished studies. While there is a trade-off between transport capacity and trips required per cell, the highest provisioning rates are achieved by bees carrying very large pollen loads at intermediate trip durations. Most solitary bees appear to be either egg or resource limited, so sustained provisioning rates over one cell per day are unusual. Provisioning rate / body size / transport capacity / solitary bees / trip duration 1. INTRODUCTION tradeoffs between provisioning one large cell or two small cells direct, indirect, or nonex- There is a vast literature on the foraging tac- istent? Are provisioning rates related to body tics of bees: what kinds of flowers to visit, how size? long to spend on a flower, how many flowers Provisioning rate is a general term and can in an inflorescence to visit, when to turn, and simply refer to the rate (mass per unit time) so forth. Much of this work is done within a at which foragers bring various food items framework of whether a forager should max- (pollen, nectar, oils, carrion, or whatever) to imize harvesting rate or foraging efficiency their nests. -

Hymenoptera, Apoidea)
>lhetian JMfuseum ox4tates PUBLISHED BY THE AMERICAN MUSEUM OF NATURAL HISTORY CENTRAL PARK WEST AT 79TH STREET, NEW YORK 24, N.Y. NUMBER 2 2 24 AUGUST I7, I 965 The Biology and Immature Stages of Melitturga clavicornis (Latreille) and of Sphecodes albilabris (Kirby) and the Recognition of the Oxaeidae at the Family Level (Hymenoptera, Apoidea) BYJEROME G. ROZEN, JR.' Michener (1944) divided the andrenid subfamily Panurginae into two tribes, the Panurgini and Melitturgini, with the latter containing the single Old World genus Melitturga. This genus was relegated to tribal status apparently on the grounds that the adults, unlike those of other panurgines, bear certain striking resemblances to the essentially Neo- tropical Oxaeinae of the same family. In 1951 Rozen showed that the male genitalia of Melitturga are unlike those of the Oxaeinae and are not only typical of those of the Panurginae in general but quite like those of the Camptopoeum-Panurgus-Panurginus-Epimethea complex within the sub- family. On the basis of this information, Michener (1954a) abandoned the idea that the genus Melitturga represents a distinct tribe of the Panur- ginae. Recently evidence in the form of the larva of Protoxaea gloriosa Fox (Rozen, 1965) suggested that the Oxaeinae were so unlike other Andreni- dae that they should be removed from the family unless some form inter- 1 Chairman and Associate Curator, Department of Entomology, the American Museum of Natural History. 2 AMERICAN MUSEUM NOVITATES NO. 2224 mediate between the two subfamilies is found. In spite of the structure of the male genitalia, Melitturga is the only known possible intermediary.