Metals Handbook VOL 15
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(Manufacturing Technology) (ATMT) - Cuyahoga Community College 2021-2022 Catalog 1
Applied Industrial Technology (Manufacturing Technology) (ATMT) - Cuyahoga Community College 2021-2022 Catalog 1 ATMT-1200 Machine Tool Theory APPLIED INDUSTRIAL 4 Credits Presents foundation for study of manufacturing methods, processes, TECHNOLOGY related equipment, and tools of industry, requiring student to understand shop safety practices, job planning, feeds and speeds, layout tools and (MANUFACTURING procedures, hand tools and bench work, metal cutting saws, drilling machines, lathe, milling machines, jig bore and jig grinder, surface grinder, TECHNOLOGY) (ATMT) E.D.M, and abrasives. Lecture: 4 hours ATMT-1000 Mechanical & Spatial Relations Prerequisite(s): Departmental approval: admission to Applied Industrial 4 Credits Technology - Manufacturing Technology program. Relationship between two-view and three-view images. Basics of ATMT-1300 Manufacturing Procedures visualizing three-dimensional objects from two-dimensional front, 2 Credits side, and top views. Perceptual ability, spatial views, matching parts Principles of blanking and/or piercing dies; bending; screw and dowel and figures. Visualization of shapes or patterns that can result from holes; die life; punches; pilots; die block construction; strippers and fitting together cut-up pieces. Graphically describing size and shape to stock guides; shredders and knockouts; nest gages; pushers; die stops; represent basic mechanical elements along with cube counting. stock material utilization; strip layouts; and die sets. Includes techniques Lecture: 4 hours and theory of building -

Chapter 11: Metal Casting Processes and Equipment
Manufacturing Engineering Technology in SI Units, 6th Edition Chapter 11: Metal Casting Processes and Equipment Copyright © 2010 Pearson Education South Asia Pte Ltd Chapter Outline ¨ Introduction ¨ Expendable-mold, Permanent-pattern Casting Processes ¨ Expendable-mold, Expendable-pattern Casting Processes ¨ Permanent-mold Casting Processes ¨ Casting Techniques for Single-crystal Components ¨ Rapid Solidification ¨ Inspection of Castings ¨ Melting Practice and Furnaces ¨ Foundries and Foundry Automation Copyright © 2010 Pearson Education South Asia Pte Ltd Introduction ¨ Various casting processes developed over time to meet specific design requirements Copyright © 2010 Pearson Education South Asia Pte Ltd Introduction ¨ Molding categories: 1. Expendable molds 2. Permanent molds 3. Composite molds Copyright © 2010 Pearson Education South Asia Pte Ltd Introduction ¨ General characteristics of sand casting and casting processes are summarized Copyright © 2010 Pearson Education South Asia Pte Ltd Expendable-mold, Permanent-pattern Casting Processes: Sand Casting ¨ Most prevalent form of casting ¨ Application for machine bases, large turbine impellers, propellers, plumbing fixtures Copyright © 2010 Pearson Education South Asia Pte Ltd Expendable-mold, Permanent-pattern Casting Processes: Sand Casting Sand ¨ Sand-casting operations use silica sand as the mold material ¨ Sand is inexpensive and suitable high melting point process ¨ 2 types of sand: naturally bonded (bank sand) and synthetic (lake sand) ¨ Fine grained sand enhances mold strength and lower mold permeability Copyright © 2010 Pearson Education South Asia Pte Ltd Expendable-mold, Permanent-pattern Casting Processes: Sand Casting Types of Sand Molds 3 basic types: 1. Green-sand mold Sand in the mold is moist or damp while the metal is being poured into it 2. Cold-box mold Organic and inorganic binders are blended into the sand to bond the grains chemically 3. -

The One-Shot Casting Process What Is One-Shot Casting?
The One-Shot Casting Process What is One-Shot Casting? Plaster mold casting is a method of producing aluminum, zinc or magnesium castings by pouring liquid metal into plaster (gypsum) molds. At Armstrong Mold, there are two methods of plaster mold casting: 1) Rubber Plaster Molding (RPM): Patterns create foundry tooling that makes copes, drags and core boxes used to create the plaster molds. 2) One-Shot Casting: Plaster is poured directly on patterns, which are melted out in a furnace cycle. Following is the process for the One-Shot method of making plaster mold castings. Step 1: Model/Pattern 1) Constructed from customer drawing or CAD file. 2) Laser-sintered patterns are produced. 3) Model is engineered to include: A) Metal shrinkage. B) Mold taper (if required) C) Machine stock (if required). 4) We can "clone" or adapt customer-supplied model if requested. Step 2: Plaster Mold 1) A liquid plaster slurry is poured around the pattern. 2) The plaster mold is heated in a furnace to melt out the pattern and cure plaster. Step 3: Pour Casting 1) Molten metal is prepared by degassing, and a spectrographic sample is taken to check the chemical analysis. 2) The molten metal is then poured into the plaster mold. 3) The plaster is removed by mechanical knock-out and high pressure waterjet. 4) When the casting has cooled, the gates and risers are then removed. Step 4: Secondary Operations 1) The raw castings are inspected and serialized. 2) Castings may then require (per customer specifications): A) Heat treatment B) X-Ray C) Penetrant inspection 3) After finish inspection, casting is ready for: A) Machining B) Chemical film, chromate conversion, paint special finishes D) Assembly E) Form-in-place gasketing. -

Master Mold Builder Certification Assessment Blueprint
Assessment Blueprint AMBA Master Moldmaker Certification Test Code: 8281 Version: 01 AMBA Master Moldmaker Certification Specific Competencies and Skills Tested in this Assessment: Advanced Planning and Review • Redline a mold design • Identify mold manufacturing cost issues Mold Design • Check mold components for fit and function • Design jigs and fixtures Plastic Injection Molding • Describe the process of plastic injection molding • Define functions of the plastic injection mole • Describe injection molded part features • Describe injection molded part defects • Control mold temperature • Describe parts and functions of the cavity/core assembly • Describe the plastic delivery system • Describe mold locating and spotting • Describe mold ejection systems Alternative Molding Process • Describe blow molding • Describe extrusion molding processes • Describe plastic/foam molding processes • Describe rotational molding process • Describe thermoform molding process • Describe metal injection molding • Define the die-casting process Page 1 of 5 AMBA Master Moldmaker Certification Specific Competencies and Skills continued: CAD/CAM and other Software • Use CAD software to determine undocumented dimensions • Use CAD software to troubleshoot • Demonstrate concepts of programming using CAM software • Demonstrate knowledge of communication software • Use business production software Inspection and Measurement • Request and verify part dimensions and features data • Perform in-process checks Assembly, Fit and Finish • Bench and polish steel • Perform -
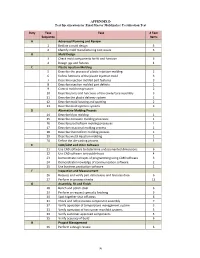
APPENDIX D Test Specification for Final Master Moldmaker Certification Test
APPENDIX D Test Specification for Final Master Moldmaker Certification Test Duty Task Task # Test Sequence Items A Advanced Planning and Review 1 Redline a mold design 5 2 Identify mold manufacturing cost issues 3 B Mold Design 3 Check mold components for fit and function 6 4 Design jigs and fixtures 4 C Plastic Injection Molding 5 Describe the process of plastic injection molding 2 6 Define functions of the plastic injection mold 3 7 Describe injection molded part features 3 8 Describe injection molded part defects 2 9 Control mold temperature 2 10 Describe parts and functions of the cavity/core assembly 3 11 Describe the plastic delivery system 2 12 Describe mold locating and spotting 2 13 Describe mold ejection systems 3 D Alternative Molding Process 14 Describe blow molding 1 15 Describe extrusion molding processes 1 16 Describe plastic/foam molding processes 1 17 Describe rotational molding process 1 18 Describe thermoform molding process 1 19 Describe metal injection molding 1 20 Define the die‐casting process 1 E CAD/CAM and other Software 21 Use CAD software to determine undocumented dimensions 6 22 Use CAD software to troubleshoot 4 23 Demonstrate concepts of programming using CAM software 3 24 Demonstrate knowledge of communication software 2 25 Use business production software 1 F Inspection and Measurement 26 Request and verify part dimensions and features data 6 27 Perform in‐process checks 13 G Assembly, Fit and Finish 28 Bench and polish steel 6 29 Perform or request specialty finishing 3 30 Spot together shut off areas -

Foundry Industry SOQ
STATEMENT OF QUALIFICATIONS Foundry Industry SOQ TRCcompanies.com Foundry Industry SOQ About TRC The world is advancing. We’re advancing how it gets planned and engineered. TRC is a global consulting firm providing environmentally advanced and technology‐powered solutions for industry and government. From solid waste, pipelines to power plants, roadways to reservoirs, schoolyards to security solutions, clients look to TRC for breakthrough thinking backed by the innovative follow‐ through of a 50‐year industry leader. The demands and challenges in industry and government are growing every day. TRC is your partner in providing breakthrough solutions that navigate the evolving market and regulatory environment, while providing dependable, safe service to our customers. We provide end‐to‐end solutions for environmental management. Throughout the decades, the company has been a leader in setting industry standards and establishing innovative program models. TRC was the first company to conduct a major indoor air study related to outdoor air quality standards. We also developed innovative measurements standards for fugitive emissions and ventilation standards for schools and hospitals in the 1960s; managed the monitoring program and sampled for pollutants at EPA’s Love Canal Project in the 1970s; developed the basis for many EPA air and hazardous waste regulations in the 1980s; pioneered guaranteed fixed‐price remediation in the 1990s; and earned an ENERGY STAR Partner of the Year Award for outstanding energy efficiency program services provided to the New York State Energy Research and Development Authority in the 2000s. We are proud to have developed scientific and engineering methodologies that are used in the environmental business today—helping to balance environmental challenges with economic growth. -

MSL Engineering Limited Platinum Blue House 1St Floor, 18 the Avenue Egham, Surrey, TW20 9AB
SMR Final Report 121404 Purpose of Issue Rev Date of Issue Author Agreed Approved Issued for information 0 Aug 2004 SM Issued for internal comment 1 November 2004 AFD DJM JB Issued as Final Report 2 December 2004 AFD DJM JB This Final report has been reviewed and approved by the Mineral Management Service. Approval does not signify that the contents necessarily reflect the views and policies of the Service, nor does mention of trade names or commercial products constitute endorsement or recommendation for use. This study was funded by the Mineral Management Service, U.S. Department of the Interior, Washington, D.C., under Contract Number 1435-01-04-CT-35320 ASSESSMENT OF REPAIR TECHNIQUES FOR AGEING OR DAMAGED STRUCTURES Project #502 DOC REF C357R001 Rev 1 NOV 2004 MSL Engineering Limited Platinum Blue House 1st Floor, 18 The Avenue Egham, Surrey, TW20 9AB Tel: +44 (0)1784 439194 Fax: +44 (0)1784 439198 E-mail: [email protected] C357R001Rev 2, December 2004 MMS Project #502 NUMBER DETAILS OF REVISION 0 Issued for information, August 2004 1 Issued for comment, November 2004. Extensive revisions throughout, including restructuring of report. 2 Issued as Final Report, December 2004. Conversion table added, Figure showing clamp details to avoid added, and general editorial revisions. C357R001Rev 2, December 2004 MMS Project #502 Assessment of Repair Techniques for Ageing or Damaged Structures By Dr. Adrian F Dier MSL Services Corporation Final Project Report: ASSESSMENT OF REPAIR TECHNIQUES FOR AGEING OR DAMAGED STRUCTURES MMS Project Number 502 November 2004 C357R001Rev 2, December 2004 i This Final report has been reviewed a nd approved by the Mineral Management Service. -

Powder Metallurgy Review Autumn 2014
POWDER METALLURGY VOL. 3 NO. VOL. 2014 AUTUMN/FALL REVIEW KEYSTONE POWDERED METAL COMPANY HOT ISOSTATIC PRESSING HARDMETAL STANDARDS Published by Inovar Communications Ltd ipmd.net PowderMetallurgyReview Feb2014.pdf 1 1/14/14 10:02 AM Publisher & editorial offices Inovar Communications Ltd 2 The Rural Enterprise Centre POWDER Battlefield Enterprise Park Shrewsbury SY1 3FE METALLURGY United Kingdom Editor & Publishing Director REVIEW Paul Whittaker AUTOMOTIVE HEAVY INDUSTRY AEROSPACE Tel: +44 (0)1743 454992 Email: [email protected] Managing Director Nick Williams Tel: +44 (0)1743 454991 THE FUTURE OF PM SOLUTIONS Email: [email protected] Accuracy of contents A classic comeback story Whilst every effort has been made to ensure the accuracy of the information in In an upbeat presentation at the PM2014 World Congress HAS ARRIVED! this publication, the publisher accepts no responsibility for errors or omissions or in Orlando, Florida, MPIF President Richard Pfingster told for any consequences arising there from. international delegates that the Powder Metallurgy industry’s Inovar Communications Ltd cannot be held recovery since 2010 was “a classic comeback story, in The potential of powder metallurgy responsible for views or claims expressed is only limited by one’s imagination… by contributors or advertisers, which are not which an industry rallies an inner strength rooted in its long necessarily those of the publisher. tradition of entrepreneurial grit.” To that end, from developing new materials Reproduction, storage and usage that lead to property advancements or Single photocopies of articles may be made This sense of optimism, which could be felt in both the PM process eciency, to working with for personal use in accordance with national PM2014 congress sessions and in the exhibition hall, was copyright laws. -

Thermoforming Quarterly ®
Thermoforming ® Quarterly A JOURNAL OF THE THERMOFORMING DIVISION OF THE SOCIETY OF PLASTICS ENGINEERS THIRD QUARTER 2015 n VOLUME 34 n NUMBER 3 Forming Insights with Data Thermoformed Packaging 2015 pages 8-9 INSIDE … Improvements in Color Technology pages 28-29 2015 Thermoformer of the Year page 30 2015 Conference Preview pages 38-42 WWW.THERMOFORMINGDIVISION.COM ARE SCREAMIN PEOPLE G “M ORE PMC!” PREMIER MATERIAL CONCEPTS UUCCIINNGG...... ROODD NNTTR E BEEAASST II FF TTHHE B T OO HEE NN TTH SSOO The Son of the Beast, PMC’s new state-of-the-art extrusion line, offers increased capacity and expanded production flexibility. We’re hungry for your business. Call today to place your order. STARRING AMY HOYLE JIM MCVICAR JR HOPPENJANS 838.429.7586 [email protected] 567.525.1577 [email protected] 419.306.2394 [email protected] GREG HORTON BILL BUSSER STEVEN ZAGAMI 913.522.6255 [email protected] 419.889.5954 [email protected] 419.890.6334 [email protected] 877.289.7626 // [email protected] // buypmc.com 2 THERMOFORMING QUARTERLY Thermoforming THIRD QUARTER 2015 Quarterly® VOLUME 34 n NUMBER 3 Thermoforming Quarterly® n Departments A JOURNAL PUBLISHED EACH CALENDAR QUARTER BY THE Chairman’s Corner x 4 THERMOFORMING DIVISION Thermoforming in the News x 6 OF THE SOCIETY OF The Business of Thermoforming x 8-11 PLASTICS ENGINEERS Thermoforming and Sustainability x 44-46 Editor Conor Carlin n Features (617) 771-3321 Thermoforming 2.0: Problems I Wish I Could Solve x 18-19 [email protected] Lead Technical Article: Comparison Between Thermoform -

A New Ceramic Casting Mold Made by Gel Casting Using Silica Sol As a Binder
BFSZU Zawrah et al. Vol.38-Dec.2016 A NEW CERAMIC CASTING MOLD MADE BY GEL CASTING USING SILICA SOL AS A BINDER Mahmoud F. Zawrah (1), Sayed A. Abdullah (2), Reham M. Khattab (1), Ibrahim M. Ibrahim (2), Waleed F. Youssef (3) (1) National Research Center, Department of Ceramics. (2) Shoubra Faculty of Engineering, Benha University, Department of Mechanical Engineering. (3) Faculty of Engineering, Sinai University, Department of Mechanical Engineering. ABSTRACT This Paper presents a new ceramic casting mold made by gel casting using silica sol as a binder. The new ceramic mold is consisted of an alumina- mullite-zirconia matrix with the ratios of 38.332 wt. % alumina, 34.378 wt. % mullite, and 27.294 wt. % zirconia respectively, the slurry is mixed then the gelling agent is added and poured into the pattern. After gelation the mold is extracted and left to dry, then sintered. There are three main defects appear in the mold fabrication process. The 1st defect is the mold cracking, as a result of forced shrinkage of mold into pattern. The 2nd defect is the bad gelation behavior of mold, as a result of non equal gelling time. The last defect is mold surface cracks, due to increased silica ratio added to the mixture. As zirconia increased the bulk density and apparent porosity is increased, leading to higher mold permeability which is important to eliminate trapping of residual gases. The increased zirconia content decreases the micro hardness and the cold crushing strength, but increases the thermal shock resistance due to phase transformation during sintering. The ceramic mold is applicable for nodular cast iron so that the mold is hard enough to withstand the forces of spheroidal graphite formation when nodular cast iron is poured into the ceramic mold. -

Injection Molding WIKIPEDIA
Design and manufacturing of plastic injection mould Content Design and manufacturing of plastic injection mould ............................................................... 1 1 Injection molding ............................................................................................................... 3 1.1 History........................................................................................................................ 3 1.2 Equipment .................................................................................................................. 3 1.3 Injection molding cycle.............................................................................................. 6 1.4 Molding trial............................................................................................................... 7 1.5 Molding defects.......................................................................................................... 7 2 Injection molding machine............................................................................................... 10 2.1 Types of injection molding machines ...................................................................... 10 2.2 Injection unit ............................................................................................................ 10 2.3 Clamping unit........................................................................................................... 12 3 Injection mould ............................................................................................................... -

Manufacturing Technology I Unit I Metal Casting
MANUFACTURING TECHNOLOGY I UNIT I METAL CASTING PROCESSES Sand casting – Sand moulds - Type of patterns – Pattern materials – Pattern allowances – Types of Moulding sand – Properties – Core making – Methods of Sand testing – Moulding machines – Types of moulding machines - Melting furnaces – Working principle of Special casting processes – Shell – investment casting – Ceramic mould – Lost Wax process – Pressure die casting – Centrifugal casting – CO2 process – Sand Casting defects. UNIT II JOINING PROCESSES Fusion welding processes – Types of Gas welding – Equipments used – Flame characteristics – Filler and Flux materials - Arc welding equipments - Electrodes – Coating and specifications – Principles of Resistance welding – Spot/butt – Seam – Projection welding – Percusion welding – GS metal arc welding – Flux cored – Submerged arc welding – Electro slag welding – TIG welding – Principle and application of special welding processes – Plasma arc welding – Thermit welding – Electron beam welding – Friction welding – Diffusion welding – Weld defects – Brazing – Soldering process – Methods and process capabilities – Filler materials and fluxes – Types of Adhesive bonding. UNIT III BULK DEFORMATION PROCESSES Hot working and cold working of metals – Forging processes – Open impression and closed die forging – Characteristics of the process – Types of Forging Machines – Typical forging operations – Rolling of metals – Types of Rolling mills – Flat strip rolling – Shape rolling operations – Defects in rolled parts – Principle of rod and wire drawing – Tube drawing – Principles of Extrusion – Types of Extrusion – Hot and Cold extrusion – Equipments used. UNIT IV SHEET METAL PROCESSES Sheet metal characteristics – Typical shearing operations – Bending – Drawing operations – Stretch forming operations –– Formability of sheet metal – Test methods – Working principle and application of special forming processes – Hydro forming – Rubber pad forming – Metal spinning – Introduction to Explosive forming – Magnetic pulse forming – Peen forming – Super plastic forming.