TLR4-Mediated Expulsion of Bacteria from Infected Bladder Epithelial Cells
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Abl Family Tyrosine Kinases Govern Igg Extravasation in the Skin in a Murine Pemphigus Model
ARTICLE https://doi.org/10.1038/s41467-019-12232-3 OPEN Abl family tyrosine kinases govern IgG extravasation in the skin in a murine pemphigus model Sachiko Ono1, Gyohei Egawa1, Takashi Nomura1, Akihiko Kitoh1, Teruki Dainichi 1, Atsushi Otsuka1, Saeko Nakajima1, Masayuki Amagai2, Fumi Matsumoto3, Mami Yamamoto 3, Yoshiaki Kubota4, Toshiyuki Takai5, Tetsuya Honda1 & Kenji Kabashima 1,6 1234567890():,; The pathway of homeostatic IgG extravasation is not fully understood, in spite of its importance for the maintenance of host immunity, the management of autoantibody- mediated disorders, and the use of antibody-based biologics. Here we show in a murine model of pemphigus, a prototypic cutaneous autoantibody-mediated disorder, that blood- circulating IgG extravasates into the skin in a time- and dose-dependent manner under homeostatic conditions. This IgG extravasation is unaffected by depletion of Fcγ receptors, but is largely attenuated by specific ablation of dynamin-dependent endocytic vesicle for- mation in blood endothelial cells (BECs). Among dynamin-dependent endocytic vesicles, IgG co-localizes well with caveolae in cultured BECs. An Abl family tyrosine kinase inhibitor imatinib, which reduces caveolae-mediated endocytosis, impairs IgG extravasation in the skin and attenuates the murine pemphigus manifestations. Our study highlights the kinetics of IgG extravasation in vivo, which might be a clue to understand the pathological mechanism of autoantibody-mediated autoimmune disorders. 1 Department of Dermatology, Kyoto University Graduate School of Medicine, Kyoto, Japan. 2 Department of Dermatology, Keio University Graduate School of Medicine, Tokyo, Japan. 3 Research Unit/Immunology & Inflammation, Sohyaku, Innovative Research Division, Mitsubishi Tanabe Pharma Corporation, Yokohama, Japan. 4 Department of Anatomy, Keio University School of Medicine, Tokyo, Japan. -

The Mir-199–Dynamin Regulatory Axis Controls Receptor-Mediated Endocytosis Juan F
© 2015. Published by The Company of Biologists Ltd | Journal of Cell Science (2015) 128, 3197-3209 doi:10.1242/jcs.165233 RESEARCH ARTICLE The miR-199–dynamin regulatory axis controls receptor-mediated endocytosis Juan F. Aranda1,2, Alberto Canfrán-Duque1,2, Leigh Goedeke1,2, Yajaira Suárez1,2 and Carlos Fernández-Hernando1,2,* ABSTRACT mechanism for the selective uptake of essential nutrients such as Small non-coding RNAs (microRNAs) are important regulators of low-density lipoprotein (LDL), through the LDL receptor (LDLR) gene expression that modulate many physiological processes; (Brown and Goldstein, 1986), or iron, through transferrin receptor however, their role in regulating intracellular transport remains (TfR) (Harding et al., 1983). Thus, factors that affect RME have a largely unknown. Intriguingly, we found that the dynamin (DNM) direct effect on these receptors, and, in the case of LDLR, to regulate genes, a GTPase family of proteins responsible for endocytosis in intracellular cholesterol levels. In both the LDLR and TfR eukaryotic cells, encode the conserved miR-199a and miR-199b internalization processes, clathrin plays a key role during the family of miRNAs within their intronic sequences. Here, we formation of coated vesicles (Moore et al., 1987). Once vesicles are demonstrate that miR-199a and miR-199b regulate endocytic internalized, their passage through a broad endosomal compartment transport by controlling the expression of important mediators of system is required; first they are rapidly transported into early endocytosis such as clathrin heavy chain (CLTC), Rab5A, low- endosomes, where Rab5A is a key regulator (Nielsen et al., 1999), density lipoprotein receptor (LDLR) and caveolin-1 (Cav-1). -

Palmitoylation of Caveolin-1 and Its Importance for Structural and Functional Plasticity
Palmitoylation of Caveolin-1 and its importance for structural and functional plasticity A DISSERTATION SUBMITTED TO THE FACULTY OF UNIVERSITY OF MINNESOTA BY Katherine R. Tonn IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY Paul G. Mermelstein November 2018 © Katherine R. Tonn Eisinger 2018 Acknowledgments I would like to thank my adviser, Dr. Paul Mermelstein, for his mentorship, training, support, and scientific flexibility. Infinite thanks to past and present members of the Mermelstein and Meisel Labs, especially Dr. Brittni Peterson, Dr. Kelsey Moore, Dr. Laura Been, Dr. Luis Martinez, Dr. Valerie Hedges, and Dr. John Meitzen, for being a source of scientific inspiration and friendship. I could not have asked for a better friend and lab colleague than Dr. Kellie Gross, whose input has kept me sane and made me a better scientist. Thanks also to stellar undergrads Kerry Trotter, Julia Dworsky, and Sam Swanson for their help and energy. Thanks to the many people who collaborated or helped in some way on the experiments presented in this thesis, including Dr. Mark Thomas, Dr. Lorene Lanier, Dr. Mark Dell’Acqua, Dr. Kevin Woolfrey, Dr. Brian Head, Dr. Jing Tong, and Dr. John Meitzen. In particular, I am extremely grateful for the collaboration of Dr. Lorene Lanier, whose enthusiasm helped reinvigorate me and this project. Finally, thanks to the members of my thesis committee, Drs. Harry Orr, Timothy Ebner, Anna Lee, and Robert Meisel, for their encouragement and guidance. i Dedication For Mom, my first and most important example of what it is to be smart, versatile, and kind. -

Role of Stromal Caveolin-1 (CAV1) Levels in Breast Cancer Angiogenesis
Universidad Autónoma de Madrid Programa de Doctorado en Biociencias Moleculares Role of stromal Caveolin-1 (CAV1) levels in breast cancer angiogenesis Alberto Díez Sánchez Madrid, 2018 0 1 Departamento de Bioquímica Facultad de Medicina Universidad Autónoma de Madrid Role of stromal Caveolin-1 (CAV1) levels in breast cancer angiogenesis Doctorando: Alberto Díez Sánchez, Licenciado en Biotecnología Director: Miguel Ángel del Pozo Barriuso, MD, PhD. Fundación Centro Nacional de Investigaciones Cardiovasculares Carlos III (CNIC) Madrid, 2018 1 2 CERTIFICADO DEL DIRECTOR DE TESIS El doctor Miguel Ángel del Pozo Barriuso CERTIFICA que el doctorando Alberto Díez Sánchez ha desarrollado y concluido su trabajo de tesis doctoral “Role of stromal Caveolin-1 (CAV1) levels in breast cancer angiogenesis” bajo su supervisión, en el Centro Nacional de Investigaciones Cardiovasculares (CNIC). Y para que así conste lo firma en Madrid, a 10 de Julio de 2018, Fdo. Dr. Miguel Ángel del Pozo Barriuso Centro Nacional de Investigaciones Cardiovasculares (CNIC) 3 4 ACKNOWLEDGMENTS It is said that scientific knowledge is built on top of the shoulder of giants, in more practical terms, I consider all these people below my personal giants. First ones I encountered, were my parents and grandparents, everything I have achieved has been done on top of their previous efforts, to them I dedicate my most sincere gratitude for teaching this once lazy kid the value of effort. Next, I have to thank all those high-school teachers and university professors that during my education have been able to spark in me the sense of amazement derived from understanding how nature works. -

Novel Missense Mutation in the Caveolin-3 Gene in a Belgian Family
1349 J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.2003.028217 on 16 August 2004. Downloaded from SHORT REPORT Novel missense mutation in the caveolin-3 gene in a Belgian family with rippling muscle disease P Y K Van den Bergh, J M Ge´rard, J A Elosegi, M U Manto, C Kubisch, B G H Schoser ............................................................................................................................... J Neurol Neurosurg Psychiatry 2004;75:1349–1351. doi: 10.1136/jnnp.2003.028217 too small’’). The patient was diagnosed as having fibromyal- Rippling muscle disease (RMD) is a rare muscle disorder gia, which led to secondary depression and loss of her job. characterised by muscle stiffness, exercise induced myalgia, Muscle weakness and pigmenturia were absent. The medical and cramp-like sensations. It is genetically heterogeneous history was remarkable for mild hypothyroidism, peptic and can be acquired, but most cases show autosomal oesophagitis, and tobacco related asthmatiform bronchitis dominant inheritance due to mutations in the caveolin-3 with emphysema. At age 38, she had a neurological work-up. (CAV3) gene. We report a novel heterozygous missense The cranial nerves, muscle bulk, muscle strength, and deep mutation in CAV3 in a Belgian family with autosomal tendon reflexes were normal, and plantar responses were dominant RMD. flexor. Tapping with the reflex hammer or with the finger on A 40 year old woman complained of fatigue, exercise the muscles provoked an immediate, short lasting, forceful induced muscle pain, and muscle cramps since the age of 35. contraction and/or painful local mounding lasting for several Neurological examination revealed percussion induced seconds. PIRCs were most pronounced in the sternocleido- rapid muscle contractions (PIRCs) and localised muscle mastoid, deltoid, biceps, brachioradialis, finger and wrist mounding on percussion; muscle rippling was not observed. -
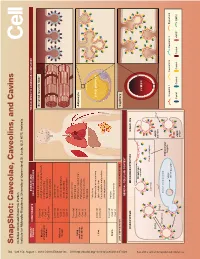
Snapshot: Caveolae, Caveolins, and Cavins Nicholas Ariotti and Robert G
704 Cell SnapShot: Caveolae, Caveolins, and Cavins Nicholas Ariotti and Robert G. Parton 154 Institute for Molecular Bioscience, University of Queensland, St. Lucia, QLD 4072, Australia , August 1,2013©2013 Elsevier Inc. DOI http://dx.doi.org/10.1016/j.cell.2013.07.009 ORGAN/ COMPONENTS DISEASE AND TISSUE-SPECIFIC CAVEOLAR COMPLEXES TISSUE CELLULAR PROCESS Rippling muscle disease Caveolin3 Striated muscle fiber Limb girdle muscular dystrophy Muscle Cavin1 skeletal and Cardiomyopathy Cavin4 cardiac Mechanoprotection Cav1 (heart) T-tubule formation Caveolin1 Lipodystrophy Caveolin2 Lipotoxicity Adipose Cavin1 Fatty acid regulation tissue Cavin2 Insulin signaling Cavin3 Mechanoprotection Atherosclerosis Caveolin1 Inammation Caveolin2 Lung Pulmonary hypertension and other Cavin1 Adipocyte Pulmonary brosis endothelia Cavin2 Mechanosensation Cavin3 Signaling Fatty liver Hepatocellular carcinoma Caveolin1 LIPID DROPLET Liver Lipid metabolism Caveolin2 Carbohydrate metabolism Liver regeneration Caveolin1 Autism Brain Cavin1 Schizophrenia Cavin3 LOW EXPRESSION HIGH EXPRESSION Capillary GENERAL CELLULAR CONTEXT ENDOCYTOSIS MECHANOPROTECTION SIGNALING See online version for legend and references. and legend for version online See Membrane stretch LUMEN Cavin complex Caveola EHD2 eNOS Ca2+ inactive Rab5 Dynamin2 EARLY ENDOSOME Intracellular targets MVB/ Caveolin 1 Caveolin 2 Caveolin 3 Dynamin2 late endosome eNOS Cavin1 Cavin2 Cavin3 Cavin4 eNOS EHD2 active SnapShot: Caveolae, Caveolins, and Cavins Nicholas Ariotti and Robert G. Parton Institute for Molecular Bioscience, University of Queensland, St. Lucia, QLD 4072, Australia Caveolae, submicroscopic bulb-shaped plasma membrane pits, are an abundant feature of many mammalian cells (Parton and del Pozo, 2013). Caveolae and the major proteins of caveolae, caveolins (Rothberg et al., 1992), and cavins (Hill et al., 2008), are linked to a number of human diseases such as muscular dystrophy, cardiomyopathy, and lipodys- trophy (see Glossary) (Bruno et al., 1993; Fernández et al., 2006; Hayashi et al., 2009). -

Comprehensive Analysis of Lncrna‑Associated Cerna Network Reveals the Novel Potential of Lncrna, Mirna and Mrna Biomarkers in Human Rectosigmoid Junction Cancer
ONCOLOGY LETTERS 21: 144, 2021 Comprehensive analysis of lncRNA‑associated ceRNA network reveals the novel potential of lncRNA, miRNA and mRNA biomarkers in human rectosigmoid junction cancer QIANSHI ZHANG1*, ZHEN FENG1*, SHASHA SHI2, YU ZHANG3 and SHUANGYI REN1 Departments of 1Gastrointestinal Surgery, 2Ultrasound and 3Clinical Laboratory, The Second Affiliated Hospital of Dalian Medical University, Dalian, Liaoning 116023, P.R. China Received March 11, 2020; Accepted November 20, 2020 DOI: 10.3892/ol.2020.12405 Abstract. Although accumulating evidence has confirmed gene 20) and three mRNAs (sodium‑ and chloride‑dependent the potential biological functions of long non‑coding RNAs taurine transporter, fibroblast growth factor 13 and tubulin (lncRNAs) as competitive endogenous RNAs (ceRNAs) in polyglutamylase TTLL7) were significantly associated with colorectal tumorigenesis and progression, few studies have OS (P<0.05). Additionally, two lncRNAs (KCNQ1OT1 focused on rectosigmoid junction cancer. In the present and MIR17HG) interacted with most of the DEmiRNAs. study, a comprehensive analysis was conducted to explore Notably, two top‑ranked miRNAs (hsa‑miR‑374a‑5p and lncRNA‑mediated ceRNA implications and their potential hsa‑miR‑374b‑5p) associated networks were identified to be value for prognosis. lncRNA, microRNA (miR/miRNA) markedly associated with the pathogenesis. Furthermore, four and mRNA expression profiles were downloaded from DEmRNAs (caveolin‑1, MET, filamin‑A and AKT3) were The Cancer Genome Atlas database. Subsequently, a enriched in the Kyoto Encylopedia of Gene and Genomes lncRNA‑miRNA‑mRNA regulatory network was constructed pathway analysis, as well as being included in the ceRNA to evaluate the functions of these differentially expressed genes network. In summary, the present results revealed that a on overall survival (OS) for rectosigmoid junction cancer. -

Caveolar Endocytosis of Simian Virus 40 Reveals a New Two-Step Vesicular- Transport Pathway to the ER
articles Caveolar endocytosis of simian virus 40 reveals a new two-step vesicular- transport pathway to the ER Lucas Pelkmans*, Jürgen Kartenbeck† and Ari Helenius*‡ *Institute of Biochemistry, Swiss Federal Institute of Technology, Universitaetstrasse 16, CH-8092 Zürich, Switzerland †German Cancer Research Center (DKFZ) Heidelberg, Im Neuenheimer Feld 280, D-69120 Heidelberg, Germany ‡e-mail: [email protected] Simian virus 40 (SV40) is unusual among animal viruses in that it enters cells through caveolae, and the internalized virus accumulates in a smooth endoplasmic reticulum (ER) compartment. Using video-enhanced, dual-colour, live fluorescence microscopy, we show the uptake of individual virus particles in CV-1 cells. After associating with cave- olae, SV40 leaves the plasma membrane in small, caveolin-1-containing vesicles. It then enters larger, peripheral organelles with a non-acidic pH. Although rich in caveolin-1, these organelles do not contain markers for endo- somes, lysosomes, ER or Golgi, nor do they acquire ligands of clathrin-coated vesicle endocytosis. After several hours in these organelles, SV40 is sorted into tubular, caveolin-free membrane vesicles that move rapidly along microtubules, and is deposited in perinuclear, syntaxin 17-positive, smooth ER organelles. The microtubule-disrupt- ing agent nocodazole inhibits formation and transport of these tubular carriers, and blocks viral infection. Our results demonstrate the existence of a two-step transport pathway from plasma-membrane caveolae, through an intermediate organelle (termed the caveosome), to the ER. This pathway bypasses endosomes and the Golgi com- plex, and is part of the productive infectious route used by SV40. any animal viruses take advantage of receptor-mediated mutants of caveolin-3 localize to intracellular vesicles that are dis- endocytosis to enter their host cells. -

Starting a Molecular Systems View of Endocytosis
ANRV356-CB24-20 ARI 3 September 2008 19:11 ANNUAL Protein Kinases: Starting REVIEWS Further Click here for quick links to Annual Reviews content online, a Molecular Systems View including: • Other articles in this volume of Endocytosis • Top cited articles • Top downloaded articles • Our comprehensive search Prisca Liberali, Pauli Ram¨ o,¨ and Lucas Pelkmans Institute of Molecular Systems Biology, ETH Zurich, CH-8093 Zurich, Switzerland; email: [email protected] Annu. Rev. Cell Dev. Biol. 2008. 24:501–23 Key Words First published online as a Review in Advance on membrane trafficking, phosphorylation, signal transduction, July 3, 2008 complexity, nonlinear systems, genetical physics The Annual Review of Cell and Developmental Biology is online at cellbio.annualreviews.org Abstract This article’s doi: The field of endocytosis is in strong need of formal biophysical model- 10.1146/annurev.cellbio.041008.145637 ing and mathematical analysis. At the same time, endocytosis must be Copyright c 2008 by Annual Reviews. much better integrated into cellular physiology to understand the for- by Universitat Zurich- Hauptbibliothek Irchel on 04/05/13. For personal use only. All rights reserved mer’s complex behavior in such a wide range of phenotypic variations. Annu. Rev. Cell Dev. Biol. 2008.24:501-523. Downloaded from www.annualreviews.org 1081-0706/08/1110-0501$20.00 Furthermore, the concept that endocytosis provides the space-time for signal transduction can now be experimentally addressed. In this review, we discuss these principles and argue for a systematic and top-down ap- proach to study the endocytic membrane system. We provide a summary of published observations on protein kinases regulating endocytic ma- chinery components and discuss global unbiased approaches to further map out kinase regulatory networks. -
Modulation of the Caveolin-3 Localization to Caveolae and STAT3 to Mitochondria by Catecholamine-Induced Cardiac Hypertrophy in H9c2 Cardiomyoblasts
EXPERIMENTAL and MOLECULAR MEDICINE, Vol. 41, No. 4, 226-235, April 2009 Modulation of the caveolin-3 localization to caveolae and STAT3 to mitochondria by catecholamine-induced cardiac hypertrophy in H9c2 cardiomyoblasts Kyuho Jeong*, Hayeong Kwon*, amine-induced cardiac hypertrophy. Chanhee Min and Yunbae Pak1 Keywords: cardiomegaly; caveolae; caveolin-3; cell Department of Biochemistry nucleus; heart; isoproterenol; mitochondria; phenyl- Division of Applied Life Science (BK21), PMBBRC ephrine; STAT3 transcription factor Environmental Biotechnology National Core Research Center Gyeongsang National University Jinju 660-701, Korea Introduction 1Corresponding author: Tel, 82-55-751-5961; Fax, 82-55-759-9363; E-mail, [email protected] Hypertension is major risk factors for cardiac da- mage, ischemia, myocardial infarction, and conge- *These authors contributed equally to this work. stive heart failure (Zampaglione et al., 1996). In DOI 10.3858/emm.2009.41.4.025 response to increased demands for cardiac work caused by various pathologic stresses, heart adapts Accepted 20 November 2008 through compensatory hypertrophy of myocytes. Abbreviations: Akt, protein kinase B; CsA, cyclosporin A; GPCR, Thus, cardiac hypertrophy is recognized in many G protein-coupled receptor; ISO, isoproterenol; PE, phenylephrine; cardiovascular diseases, such as hypertension, RTK, receptor tyrosine kinase; STAT3, signal transducers and acti- vascular disease, and myocardial infarction, and is vator of transcription 3 an independent risk factor for cardiac morbidity and mortality. Hypertrophic stimuli induce an in- crease in cell size in the absence of cell division through Ca2+ signaling and activation of PKC, Abstract MAPK and PKB/ Akt (Watanabe et al., 2001; Dorn and Force, 2005), and are accompanied by We investigated the effect of phenylephrine (PE)- and increased protein synthesis with reprogramming of isoproterenol (ISO)-induced cardiac hypertrophy on gene expression (Takeo et al., 2000). -

Caveolin-1 Expression in Schwann Cells
GLIA 27:39–52 (1999) Caveolin-1 Expression in Schwann Cells DANIEL D. MIKOL,* HOYLOND L. HONG, HSIN-LIN CHENG, AND EVA L. FELDMAN Department of Neurology, University of Michigan, Ann Arbor, Michigan KEY WORDS caveolae; cholesterol; differentiation; myelin; nerve ABSTRACT Caveolae are non-clathrin-coated invaginations of the plasma mem- brane, which are present in most cell types. An integral component of caveolae is the caveolin family of related proteins, which not only forms the structural framework of caveolae, but also likely subserves its functional roles, including regulation of signal transduction and cellular transport, in particular, cholesterol trafficking. Although caveolae have been identified ultrastructurally in the peripheral nervous system (PNS), caveolin expression has not previously been studied. To date, three caveolin genes have been reported. Here, we show for the first time that caveolin-1 is expressed by Schwann cells (SC) as well as several SC-derived cell lines. Caveolin-1 is enriched in the buoyant, detergent-insoluble membranes of rat sciatic nerve (SN) and SC, a hallmark of the caveolar compartment. Caveolin-1 exists as both soluble and insoluble forms in rat SN and SC, and localizes to SC cytoplasm and abaxonal myelin. SC caveolin-1 decreases after axotomy, when SC revert to a premyelinating phenotype. We speculate that caveolin-1 may regulate signal transduction and/or cholesterol transport in myelinating SC. GLIA 27:39–52, 1999. 1999 Wiley-Liss, Inc. INTRODUCTION A number of functions are proposed for caveolae: (1) the uptake of small molecules such as folate into cells, a Caveolae are plasma membrane microdomains that process known as potocytosis (Anderson et al., 1992); can invaginate to form 50–100 nm vesicles, and are (2) the transcytosis of molecules such as low density enriched in cholesterol, glycosphingolipids, a variety of lipoprotein across cells (Simionescu, 1983); (3) polar- signaling molecules, and the caveolins, a family of ized trafficking of proteins, especially in epithelial cells 18–24 kD proteins. -

Caveolin-1 Expression and Cavin Stability Regulate Caveolae Dynamics in Adipocyte Lipid Store Fluctuation
4032 Diabetes Volume 63, December 2014 Nolwenn Briand,1 Cécilia Prado,1 Guillaume Mabilleau,2 Françoise Lasnier,1 Xavier Le Lièpvre,1 Jeffrey D. Covington,3 Eric Ravussin,3 Soazig Le Lay,4 and Isabelle Dugail5 Caveolin-1 Expression and Cavin Stability Regulate Caveolae Dynamics in Adipocyte Lipid Store Fluctuation Diabetes 2014;63:4032–4044 | DOI: 10.2337/db13-1961 Adipocytes specialized in the storage of energy as fat Caveolae are small flask-shaped invaginations of the plasma are among the most caveolae-enriched cell types. Loss membrane (1) that are found with remarkable abundance in of caveolae produces lipodystrophic diabetes in humans, endothelial cells, myotubes, and adipocytes. They are consid- which cannot be reversed by endothelial rescue of ered a subset of the so-called lipid raft domains and segregate caveolin expression in mice, indicating major impor- a number of membrane-related processes (2). An accepted tance of adipocyte caveolae. However, how caveolae paradigm is that caveolae formation is primarily driven by participate in fat cell functions is poorly understood. the assembly of a cytoplasmic coat consisting of oligomeric We investigated dynamic conditions of lipid store caveolins (3), a protein family with 3 highly-related members fluctuations and demonstrate reciprocal regulation of (caveolin-1 through -3). Invalidation of individual caveolin caveolae density and fat cell lipid droplet storage. We genes led to the generation of mice models lacking caveolae METABOLISM identified caveolin-1 expression as a crucial step in in all cell types or in a tissue-restricted manner (4). Caveolin- adipose cell lines and in mice to raise the density of 1–null mice, which also lack caveolin-2, suffer from severe caveolae, to increase adipocyte ability to accommodate vascular dysfunction and pulmonary defects (5,6) and de- larger lipid droplets, and to promote cell expansion velop lipodystrophy (7), a metabolic phenotype that cannot by increased glucose utilization.