Pueraria Lobata) And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

1 Taxonomy and Nomenclature 2 Origin in the United States
Kudzu For other uses, see Kudzu (disambiguation). Kudzu (/ˈkʊdzuː/, also called Japanese arrow- Kudzu seedpods are considered to be varieties rather than full species. The morphological differences between them are subtle; they can breed with each other, and it appears that intro- duced kudzu populations in the United States have ances- try from more than one of the species.*[4]*[5] They are: Flowers of Pueraria montana • P. montana root*[1]*[2]) is a group of plants in the genus Pueraria, in • P. lobata (P. montana var. lobata) the pea family Fabaceae, subfamily Faboideae. They are • climbing, coiling, and trailing perennial vines native to P. edulis much of eastern Asia, southeast Asia, and some Pacific • P. phaseoloides Islands.*[2] The name comes from the Japanese name * for the plants, kuzu (クズ or 葛 ?) , which was written • P. thomsonii*[4] (P. montana var. chinensis)*[6] “kudzu”in historical romanizations. Where these plants are naturalized, they can be invasive and are considered • P. tuberosa noxious weeds. The plant climbs over trees or shrubs and grows so rapidly that it kills them by heavy shading.*[3] The plant is edible but often sprayed with herbicides.*[3] 2 Origin in the United States Kudzu was introduced to the United States as an orna- 1 Taxonomy and nomenclature mental bush and an effortless and efficient shade producer at the Philadelphia Continental Exposition in 1876. In The name kudzu describes one or more species in the the 1930s and '40s, the vine was rebranded as a way for genus Pueraria that are closely related, and some of them farmers to stop soil erosion. -

全文本) Acceptance for Registration (Full Version)
公報編號 Journal No.: 618 公布日期 Publication Date: 06-02-2015 分項名稱 Section Name: 接納註冊 (全文本) Acceptance for Registration (Full Version) 香港特別行政區政府知識產權署商標註冊處 Trade Marks Registry, Intellectual Property Department The Government of the Hong Kong Special Administrative Region 接納註冊 (全文本) 商標註冊處處長已根據《商標條例》(第 559 章)第 42 條,接納下列商標的註冊申請。現根據《商標條 例》第 43 條及《商標規則》(第 559 章附屬法例)第 15 條,公布申請的詳情。 根據《商標條例》第 44 條及《商標規則》第 16 條,任何人擬就下列商標的註冊提出反對,須在本公告 公布日期起計的三個月內,採用表格第 T6 號提交反對通知。(例如,若果公布日期爲 2003 年 4 月 4 日,則該三個月的最後一日爲 2003 年 7 月 3 日。)反對通知須載有反對理由的陳述及《商標規則》第 16(2)條所提述的事宜。反對人須在提交反對通知的同時,將該通知的副本送交有關申請人。 有關商標註冊處處長根據商標條例(第 43 章)第 13 條/商標條例(第 559 章)附表 5 第 10 條所接納的註冊申 請,請到 http://www.gld.gov.hk/cgi-bin/gld/egazette/index.cgi?lang=c&agree=0 檢視電子憲報。 ACCEPTANCE FOR REGISTRATION (FULL VERSION) The Registrar of Trade Marks has accepted the following trade marks for registration under section 42 of the Trade Marks Ordinance (Cap. 559). Under section 43 of the Trade Marks Ordinance and rule 15 of the Trade Marks Rules (Cap. 559 sub. leg.), the particulars of the applications are published. Under section 44 of the Trade Marks Ordinance and rule 16 of the Trade Marks Rules, any person who wishes to oppose the registration of any of these marks shall, within the 3-month period beginning on the date of this publication, file a notice of opposition on Form T6. (For example, if the publication date is 4 April 2003, the last day of the 3-month period is 3 July 2003.) The notice of opposition shall include a statement of the grounds of opposition and the matters referred to in rule 16(2). -

Certificate-Halal.Pdf
APPENDIX (A) Appendix (A), is an integral part of AHF Halal Certificate # HP 3980-CH, issued to Green Jeeva LLC., located at 2610W Horizon, Ridge Pkwy, Suite 201A, Henderson, Nevada, 89052 (USA). (Effective from June 12, 2021 through June 11, 2022) No. Product Names: 1. Acacia Catechu Extract-Khadira/Catechu 2. Achyranthes aspera Extract- Apamarga 3. Acorus calamus Extract-Bach 4. Aegle marmelos Extract- Bael 5. Allium vineale Extract - Garlic 6. Alpinia galanga Extract - Galanga 7. Amla Extract- Phyllanthus emblica 8. Andographis Extract- Kalmegh 9. Manjakani Extract 10. Arjuna Extract-Terminalia arjuna 11. Ashoka Extract-Saraca indica 12. Ashwagandha Extract-Withania somnifera 13. Asparagus racemosus Extract - Shatavari 14. Azadirachta indica Extract - Neem 15. Bacopa Extract-Bacopa monnieri/Nir Brahmi 16. Bahunia Extract - Bauhinia purpurea 17. Bamboo (Silica Extract Powder) 18. Bitter Melon Extract-Momordica charantia 19. Black Cumin Extract 20. Bhringraj Extract 21. Bambusa arundinacca (Tabasir) Dry Extract 22. Banaba Extract- Legerstromia speciosa 23. Belladona Extract-Atropa belladonna 24. Berberis aristata Extract- Daru Haridra 25. Black Tea Extract-Camellia sinensis 26. Boswellia serrata Extract - Boswellia (Salakhi) 27. Bromelain - Ananus comosus 28. Calendula officinalis Extract- Marigold 29. Camellia sinensis Extract-Tea 30. Cassia angustifolia Extract - Senna 31. Cassia fistula Extract - Amaltas 32. Cedrus deodara Extract- Deodar 33. Centella asiatica Extract - Bhrami 34. Cinnamon cassia Extract - Cinnamon (Pages 2 –30) APPENDIX (A) Appendix (A), is an integral part of AHF Halal Certificate # HP 3980-CH, issued to Green Jeeva LLC., located at 2610W Horizon, Ridge Pkwy, Suite 201A, Henderson, Nevada, 89052 (USA). (Effective from June 12, 2021 through June 11, 2022) 35. -

精緻套餐 SET MENU セット 午間超值套餐 Lunch Set / スペシャルランチ
精緻套餐 SET MENU セット 午間超值套餐 Lunch Set / スペシャルランチ NT$480 套餐 Set | お一人様 頂鮮御前三味品 Ding-Xian Imperial Three-Taste Dish (Ayu sh, Asparagus, Salad) 前菜三種盛り 養生五穀海蔘粥 Healthy Cereal and Sea Cucumber Congee 五穀米とナマコのヘルシー粥 泰式清蒸海上鮮 Thai Style Steamed Seafood 鮮魚のタイ風蒸し 鮮卷綠椰青花泥 Stir-fried Cuttlesh with Mashed Broccoli イカのブロッコリーソース 府城翡翠鮮蝦卷 Tainan Shrimp Roll 台南風海老ロール揚げレタス包み 南瓜泥焗烤海鮮 Baked Seafood with Mashed Pumpkin 海鮮のかぼちゃクリームグラタン 精緻手製輕甜品 Handmade Dessert 特製デザート (以上套餐不含酒水、所有價格需酌收10%服務費) (All Menu not included Beverages and all Set Menu are subject to add 10 % service charge) (10%のサービス料及びアルコール代が上記に加算されます) 若食材因季節或天候等素影響,致使無法正常供應本公司保有調整變動菜色之權限 Adjustments to the menu may occur in accordance to seasons change 本メニューは季節によって異なる場合があります 御膳精緻套餐 Premium Set / 頂鮮御膳コース NT$780 套餐 Set | お一人様 和風鮑魚煮 Japanese Style Braised Abalone アワビの和風煮 海皇魚翅羹 Seafood Shark’s Fin Soup フカヒレのとろみスープ 清蒸海上鮮 Steamed Sea Fish 鮮魚のあっさり蒸し 府城鮮蝦卷 Tainan Shrimp Roll 台南風海老ロール揚げ 南瓜焗干貝 Braised Scallop with Pumpkin 干し貝柱のかぼちゃグラタン 主食:3選1 (Main Dishes : Selected one)(主食三者択一) 福臨小湘粽/招牌擔仔麵/鮭魚鑲米飯 Fulin Small Hunan Style Rice Dumpling/ Chef’s Danzi Noodles/Salmon Fried Rice 湖南風豚肉ちまき/おすすめ担仔麺/鮭チャーハン 臻品水果鮮 Fresh Fruit Plate 季節のフルーツ 手製輕甜品 Handmade Dessert 特製デザート ─ 以上套餐贈鮮榨果汁:西瓜原汁.招牌椰乳.奇異果原汁 任選1杯,10%服務費及酒水另計 ─ All Menu not included Beverages Free juice : Watermelon juice,Coconut milk,Kiwifruit juice (seleted one) All Menu not included Beverages and all Set Menu are subject to add 10 % service charge 以上のコースはすべてジュース付き。 スイカジュース、オリジナルココナッツミルク、キーウィフルーツジュース 択一 10%のサービス料及びアルコール代が上記に加算されます 若食材因季節或天候等素影響,致使無法正常供應本公司保有調整變動菜色之權限 Adjustments -

Food Allergens, Sensitivities, & Substitutes
BIOMEDICAL Food Allergens, Sensitivities, & Substitutes JULIE MATTHEWS, CNC AUTISM NUTRITION SPECIALIST diets involve many restrictions Food sensitivities can also trigger due to children having asthma attacks, migraine headaches, and Julie Matthews is a leading Asensitivities or allergies to a eczema. biomedical autism diet expert. variety of foods. Typically, one begins Because food allergies and sensitivities She works with parents from with gluten-free and casein-free, affect so many bodily systems, reducing around the world to help children then often branches out into soy-, them can make a significant difference recover from the symptoms of corn-, and/or egg-free, as well. All in how a child feels and behaves. Doris autism through diet intervention. this restriction can cause feelings of Rapp, MD has been studying and Julie presents at the leading overwhelm in many parents, as options treating children with allergies for many autism conferences in the US can become limited. This article will years. In her book, Is This Your Child?, and abroad, including the Defeat discuss when, why, and how to avoid Rapp describes possible symptoms of Autism Now (DAN!) Conference. food allergies/sensitivities. allergy (and sensitivity) reactions Food allergies and in toddlers: red ears and Julie has created two helpful autism diet intervention tools for sensitivities (and their cheeks, dark eye circles parents. Her book, Nourishing accompanying and bags, glassy and Hope for Autism, and her autism symptoms) are glazed eyes, bloating, nutrition education and cooking common in children belching, diarrhea DVD, Cooking to Heal, are with autism. and/or constipation, available at Julie’s website According to Dr. -

Table of Japanese Cuisine
Table of Japanese Cuisine Wakayama Prefecture 1 Catalogue of Wakayama Prefecture’s Specialties の食卓 Table of Japanese Cuisine Grand mountains, the sea and the powerful Kuroshio Current, bright sunlight, and wisdom of people living in such a wonderful environment – Wakayama Prefecture is home to a wide variety of specialties developed through the rich nature, culture, and people. 2 Climate: Blessings from the Earth and the Sea 4 Premium Wakayama 10 Wakayama’s distinctive specialties are recommended with a focus The gourmet food of Kishu comes from its bountiful nature. on safety and reliability. Food Culture: Food History Traced to “Kinokuni” 6 Kishu Ume-Plum Burger 12 A pairing of Wakayama ume-plums and hamburgers, developed Japanese cuisine originated from the enterprising spirit of people in Kishu. with overseas sales in mind. Taste of the Hometown: Honor and Pride of the Hometown 8 Wakayama Punch 13 As a kingdom of fruit, Wakayama is proud to provide this While people in the past demonstrated their wisdom, people today pursue outstanding product filled with love for our hometowns. even deeper, which underpins Japanese cuisine. < Products Catalogue > Fruit 14 Vegetables 19 Livestock Products 22 Seafood 24 Processed foods 29 Wakayama Seasonal Product Calendar 36 Share of Wakayama’s Farm and Marine Production 38 Monde Selection 39 ※ Produce amounts and other information in this brochure are based on statistics in 2016. 3 Specialties from Wakayama lend flair to the Climate “Table of Japanese Cuisine.” W AKAYAMA In Wakayama, while the climate is moderate in some parts, the deep mountains The gourmet food of generate changes in temperature in other areas. -

(12) Patent Application Publication (10) Pub. No.: US 2006/0172059 A1 Takeuchi Et Al
US 2006O172059A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2006/0172059 A1 Takeuchi et al. (43) Pub. Date: Aug. 3, 2006 (54) METHOD OF INHIBITING WATER Jun. 9, 2003 (JP)...................................... 2003-163732 CONTENT VARATION OF COMPOSITION Aug. 10, 2003 (JP)...................................... 2003-349976 AND USE THEREOF Publication Classification (76) Inventors: Kanou Takeuchi, Okayama (JP); Michio Kubota, Okayama (JP); Toshio (51) Int. Cl. Miyake, Okayama (JP) A23G 3/00 (2006.01) AOIN 63/00 (2006.01) Correspondence Address: (52) U.S. Cl. ............................................ 426/660: 504/117 BROWDY AND NEIMARK, P.L.L.C. SUTE624 NINTH 3OO STREET, NW (57) ABSTRACT WASHINGTON, DC 20001-5303 (US) The present invention has objects for providing a method for inhibiting the moisture variation in compositions, a compo (21) Appl. No.: 10/539,138 sition E. moisture variation is E. and an SNR, 1-1. inhibiting the moisture variation in compositions. The (22) PCT Filed: Dec. 15, 2003 objects are solved by providing a method for inhibiting the (86). PCT No.: PCT/UP03/16047 moisture variation in compositions comprising incorporat ing into a composition a saccharide-derivative(s) of C.C.- (30) Foreign Application Priority Data trehalose as an effective ingredient, a composition whose moisture variation is inhibited by incorporating the saccha Dec. 19, 2002 (JP)...................................... 2002-368153 ride-derivative(s), a moisture variation inhibiting agent con Mar. 7, 2003 (JP)........................................ -

Bodibeaute MT
Healthy Beauty “ In Love With Nutrition” 1 2 It is a known fact a balanced diet supports the human body to maintain its strength. Hence, owning a healthy beauty is not a secret but a clever understanding of nutrition, the science of natural ingredients coupled with scientific sound knowledge to keep your health at its optimum. 3 Did you know? Reversing and managing aging start with caring Years of research have brought Genbelle to our current for your internal organs? At Genbelle, we believe stage of near perfection. The key to our success is you know this fact. We also believe that we can help in acknowledging that raw ingredients are important you know better with our innovative products. for cells to repair & regenerate itself while the body regulates and strengthen itself with the infiltration of Literature tells us that nutrient deficiencies may essential nutrients. We know that consuming fruit and cause DNA damage; making us age faster and it can vegetable juices periodically can cleanse the body contribute to chronic diseases. The food choices and digestive system. Juice purification process too, people make daily impact how the individual looks eliminates waste and damaged cells accumulated and feels, and yet, with the fast-paced lifestyle, from years of unhealthy diet, poor digestion, stress and many are lacking in the options of consuming the environmental pollution. So, at Genbelle, we decided best food for the body. We feel many of you are to support your body by producing food supplements facing health challenges, not because of the lack that are made only from raw ingredients, designed of food intake but from the deficiencies of essential for healing the internal organs with gentler cleansing nutrients to boost your bodily functions. -
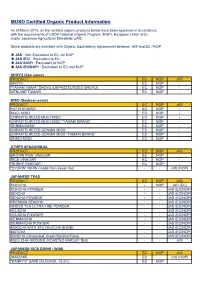
Organic Product List (PDF)
MUSO Certified Organic Product Information As of March 2015, all the certified organic products below have been approved in accordance with the requirements of USDA National Organic Program (NOP), European Union (EC), and/or Japanese Agricultural Standards (JAS). Some products are available with Organic Equivalency Agreements between JAS and EC / NOP. JAS : Non-Equivalent to EC nor NOP JAS (EC) : Equivalent to EC JAS (NOP) : Equivalent to NOP JAS (EC/NOP) : Equivalent to EC and NOP SHOYU (Soy sauce) PRODUCT EC NOP JAS SHOYU EC NOP - "YAMAKI NAMA" SHOYU (UNPASTEURIZED SHOYU) EC NOP - GENUINE TAMARI EC NOP - MISO (Soybean paste) PRODUCT EC NOP JAS HATCHO MISO EC NOP - MUGI MISO EC NOP - UNPASTEURIZED MUGI MISO EC NOP - UNPASTEURIZED MUGI MISO "YAMAKI BRAND“ EC NOP - GENMAI MISO EC NOP - UNPASTEURIZED GENMAI MISO EC NOP - UNPASTEURIZED GENMAI MISO "YAMAKI BRAND“ EC NOP - SHIRO MISO EC NOP - OTHER SEASONINGS PRODUCT EC NOP JAS BROWN RICE VINEGAR EC NOP - RICE VINEGAR EC NOP - "SUSHI" VINEGAR EC NOP - "SYURIN" MIRIN (made from sweet rice) - - JAS (NOP) JAPANESE TEAS PRODUCT EC NOP JAS KUKICHA - NOP JAS (EC) KUKICHA POWDER - - JAS (EC/NOP) SENCHA - - JAS (EC/NOP) SENCHA POWDER - - JAS (EC/NOP) PREMIUM SENCHA - - JAS (EC/NOP) GREEN TEA ULTRA FINE POWDER - - JAS (EC/NOP) HOJICHA - - JAS (EC/NOP) HOJICHA POWDER - - JAS (EC/NOP) GENMAICHA - - JAS (EC/NOP) GENMAICHA POWDER - - JAS (EC/NOP) KUKICHA WITH 20% HOJICHA BLEND - - JAS (EC/NOP) MATCHA - - JAS (EC/NOP) BANCHA (Unroasted, Green Bancha Flake) - - JAS (EC/NOP) MUGI CHA-GROUND (ROASTED BARLEY -

AKASAKA ASADA 赤坂浅田 March ,2020 ~Starter~ Slightly Blackened Fat Greenling Japanese Mountain Vegetable 'Urui' and 'Udo' Bracken
AKASAKA ASADA 赤坂浅田 March ,2020 ~Starter~ Slightly blackened fat greenling Japanese mountain vegetable 'Urui' and 'Udo' Bracken ~Seasonal appetizer~ Sea bream sushi wrapped with cherry blossom leaf Mozuku seaweed, Firefly squid boiled in sweetened soy sauce Simmered bamboo shoot, Canola blossoms Snapper roe simmered in broth, Jellied eggwash ~Soup(Owan)~ Shrimp wrapped with thin sliced daikon Bamboo shoot dumpling Bamboo shoot, Japanese pepper leaf bud ~Sashimi~ Kelp flavored Pristigenys niphonia Sweet shrimp Japanese ivory-shell ~Seasonal dish~ Sea eel and Wormwood dumpling Steamed Snow crab steeped in miso Tofu, Edible shoots Tofu, Japanese mountain vegetable 'Udo' and carrot ~Grilled dish~ Grilled Trout topped with Japanese pepper leaf bud Mixed sake lees and sesame source Deep fried edible flower bud of butterbur Marinated wasabi leaves in vinegar ~Simmered dish~ Duck and vegetable stew in traditional Kaga Style Potato, Sudare-fu, Komatsuna spinach ‘Wasabi’ ~Today’s rice or noodles~ Handmade buckwheat noodles Soba soup made from salt and kelp Or Steamed rice with bamboo shoots ~Dessert~ Seasonal Dessert AKASAKA ASADA 赤坂浅田 March ,2020 ~Starter~ Steamed clam steeped in sake Ginger vinegar Japanese mountain vegetable 'Udo' and ’Urui’ Bracken, Japanese pepper leaf bud Yolk and vinegar sauce ~Seasonal appetizer~ Large prawn and Brussels sprouts dressed with mixed sake lees and sesame sauce Sea bream sushi wrapped with cherry blossom leaf Sea cucumber ovary, Yam Firefly squid boiled in sweetened soy sauce Jellied Sea bream roe soup broth, -

This Guide Provides Specific Recommendations for Losing And
This guide provides specific recommendations for losing and sustainably managing weight.This approach relies on genetic testing for the following gene variations known to impact weight gain and appetite: • Human fat-mass and obesity associated gene (FTO) • Melanocortin 4 Receptor (MC4R) • Fatty acid binding protein 2 (FABP2) • Beta-adrenoreceptor 2 (ADRB2) • SH2B I adapter protein I (SH2B I) The food plan included in this guide relies on the primary components of a Mediterraneanstyle diet, which has been shown to have a positive impact on weight, heart health, and many other modem chronic diseased states. This guide provides an evidence-based summary of a food plan with specific macronutrient information. This guide also provides suggestions for choosing the best food plan for an individual based on his/her genetic test results. Provided is basic dietary guidance, a 7-day meal plan with recipes, and tools for creating future customized meal plans. Materials support both the weight loss phase of weight management as well as ongoing weight maintenance. 1 The food plan provided in this booklet is allele-specific and promotes a healthy eating lifestyle and has the following features: • Whole foods- whole foods are emphasized over processed and refinedfoods.Whole foods generallyprovide higher nutrient density per calorie (Drewnowski, 2005), a particularly importantfeature when reducing calorie intake for weight loss. • Minimal sugar - Sugar in all forms, even fruit, is reduced to help maintain consistent blood glucose levels, improve insulin sensitivity and reduce inflammation. Refined sugar is discouraged because of its low nutrient density. • High fiber - High fiber whole grains, vegetables and fruits make up a large part of each food plan.A high fiber eating pattern helps to promote satiety, manage blood sugar;improve insulin resistance, and remove toxins from the body through more regular bowel movements. -

Behavior Neurologic Immunity Diet Metabolism Digestion
IMMUNITY DIGESTION NUTRITION BEHAVIOR DIET NEUROLOGIC METABOLISM Underlying Predispositions Including Problems With: Gut flora, Digestion, Absorption, Utilization, Metabolism, Detoxification, Nutrition, Sulfation, Methylation, Oxidation The Total Load Diet, Lifestyle, Environment, Toxins, Medications TRIGGER THE TIPPING POINT ASD DIAGNOSIS REACTIONS TO Foods, Additives, Dyes, Toxins, Pesticides, Medications, Vaccines, Environmental Exposures 1 Remove offending foods and substances What Interferes Gluten, milk casein, soy, corn, phenols, oxalates, starches & sugars Antacids Inflammation Artificial additives, chemicals, pesticides, toxins Antibiotics Poor gut flora Pathogens Toxins Replace with Healthy foods which do not promote reactions What Helps Probiotics Omega-3 Optimize Prebiotics Vitamins A and D Nutritional status Enzymes Zinc GI flora, function, enzymes, absorption Demulscents Glutamine Sulfation, methylation, detoxification SPECIAL DIETS SPECIAL DIETS . Artificial sweeteners, preservatives, colors, flavors . High fructose corn syrup, agave . Sodas: diet and regular . Hydrogenated oils / trans fats / margarine . Refined grains and processed foods . Sugar . Caffeine . Deep fried foods . Craved foods . Food reactions 2 If it does not grow……do not eat it! FOOD ARTIFICIAL ADDITIVES, PRESERVATIVES Nutrient -Dense, Eco-Friendly Good Fats and Oils American Academy of Pediatrics Acknowledgement • Pasture-fed, grass-fed animals • Extra virgin olive oil • Eggs, seafood, meat, poultry • Butter, animal fats • Meta analysis of 15 trials concluded