HETEROPTERA:MIRIDAE) Redacted for Privacy Abstract Approved: John D
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Vol. 16, No. 2 Summer 1983 the GREAT LAKES ENTOMOLOGIST
MARK F. O'BRIEN Vol. 16, No. 2 Summer 1983 THE GREAT LAKES ENTOMOLOGIST PUBLISHED BY THE MICHIGAN EN1"OMOLOGICAL SOCIErry THE GREAT LAKES ENTOMOLOGIST Published by the Michigan Entomological Society Volume 16 No.2 ISSN 0090-0222 TABLE OF CONTENTS Seasonal Flight Patterns of Hemiptera in a North Carolina Black Walnut Plantation. 7. Miridae. J. E. McPherson, B. C. Weber, and T. J. Henry ............................ 35 Effects of Various Split Developmental Photophases and Constant Light During Each 24 Hour Period on Adult Morphology in Thyanta calceata (Hemiptera: Pentatomidae) J. E. McPherson, T. E. Vogt, and S. M. Paskewitz .......................... 43 Buprestidae, Cerambycidae, and Scolytidae Associated with Successive Stages of Agrilus bilineatus (Coleoptera: Buprestidae) Infestation of Oaks in Wisconsin R. A. Haack, D. M. Benjamin, and K. D. Haack ............................ 47 A Pyralid Moth (Lepidoptera) as Pollinator of Blunt-leaf Orchid Edward G. Voss and Richard E. Riefner, Jr. ............................... 57 Checklist of American Uloboridae (Arachnida: Araneae) Brent D. Ope II ........................................................... 61 COVER ILLUSTRATION Blister beetles (Meloidae) feeding on Siberian pea-tree (Caragana arborescens). Photo graph by Louis F. Wilson, North Central Forest Experiment Station, USDA Forest Ser....ice. East Lansing, Michigan. THE MICHIGAN ENTOMOLOGICAL SOCIETY 1982-83 OFFICERS President Ronald J. Priest President-Elect Gary A. Dunn Executive Secretary M. C. Nielsen Journal Editor D. C. L. Gosling Newsletter Editor Louis F. Wilson The Michigan Entomological Society traces its origins to the old Detroit Entomological Society and was organized on 4 November 1954 to " ... promote the science ofentomology in all its branches and by all feasible means, and to advance cooperation and good fellowship among persons interested in entomology." The Society attempts to facilitate the exchange of ideas and information in both amateur and professional circles, and encourages the study of insects by youth. -

Rainfall and Parasitic Wasp (Hymenoptera: Ichneumonoidea
Agricultural and Forest Entomology (2000) 2, 39±47 Rainfall and parasitic wasp (Hymenoptera: Ichneumonoidea) activity in successional forest stages at Barro Colorado Nature Monument, Panama, and La Selva Biological Station, Costa Rica B. A. Shapiro1 and J. Pickering Institute of Ecology, University of Georgia, Athens, GA 30602-2602, U.S.A. Abstract 1 In 1997, we ran two Malaise insect traps in each of four stands of wet forest in Costa Rica (two old-growth and two 20-year-old stands) and four stands of moist forest in Panama (old-growth, 20, 40 and 120-year-old stands). 2 Wet forest traps caught 2.32 times as many ichneumonoids as moist forest traps. The average catch per old-growth trap was 1.89 times greater than the average catch per second-growth trap. 3 Parasitoids of lepidopteran larvae were caught in higher proportions in the wet forest, while pupal parasitoids were relatively more active in the moist forest. 4 We hypothesize that moisture availability is of key importance in determining parasitoid activity, community composition and trophic interactions. Keywords Barro Colorado Nature Monument, Ichneumonoidea, La Selva, parasitoids, precipitation, tropical moist forest, tropical wet forest. istics of each parasitoid species and abiotic factors. Seasonal Introduction patterns of insect activity are often correlated with temperature, One of the largest groups of parasitic Hymenoptera is the as processes such as development and diapause are often superfamily Ichneumonoidea, which consists of two families intimately associated with temperature change (Wolda, 1988). (the Ichneumonidae and the Braconidae), 64 subfamilies and an Fink & VoÈlkl (1995) gave several examples of small insects for estimated 100 000 species world-wide (Gauld & Bolton, 1988; which low humidity and high temperature have detrimental Wahl & Sharkey, 1993). -
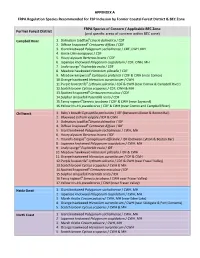
APPENDIX a FRPA Regulation Species Recommended for FSP
APPENDIX A FRPA Regulation Species Recommended for FSP Inclusion by Former Coastal Forest District & BEC Zone FRPA Species of Concern / Applicable BEC Zone Former Forest District (and specific areas of concern within BEC zone) B Campbell River 1. Dalmatian toadflax Linaria dalmatica / CDF B 2. Diffuse knapweed Centaurea diffusa / CDF 3. Giant knotweed Polygonum sachalinense / CDF, CWH, MH 4. Gorse Ulex europaeus / CDF 5. Hoary alyssum Berteroa incana / CDF 6. Japanese knotweed Polygonum cuspidatum / CDF, CWH, MH 7. Leafy spurgeB Euphorbia esula / CDF 8. Meadow hawkweed Hieracium pilosella / CDF 9. Meadow knapweedB Centaurea pratensis / CDF & CWH (near Comox) 10. Orange hawkweed Hieracium aurantiacum / CWH 11. Purple loosestrifeB Lythrum salicaria / CDF & CWH (near Comox & Campbell River) 12. Scotch broom Cytisus scoparius / CDF, CWH & MH 13. Spotted knapweedB Centaurea maculosa / CDF 14. Sulphur cinquefoil Potentilla recta / CDF 15. Tansy ragwortBSenecio jacobaea / CDF & CWH (near Sayward) 16.Yellow Iris Iris pseudacorus / CDF & CWH (near Comox and Campbell River) Chilliwack 1. Baby's breath Gypsophila paniculata / IDF (between Lillooet & Boston Bar) 2. Blueweed Echium vulgare / IDF & CWH 3. Dalmatian toadflaxBLinaria dalmatica / IDF 4. Diffuse knapweedB Centaurea diffusa / IDF 5. Giant knotweed Polygonum sachalinense / CWH, MH 6. Hoary alyssum Berteroa incana / IDF 7. Hound's-tongueB Cynoglossum officinale / IDF (between Lytton & Boston Bar) 8. Japanese knotweed Polygonum cuspidatum / CWH, MH 9. Leafy spurgeB Euphorbia esula / IDF 10. Meadow hawkweed Hieracium pilosella / IDF & CWH 11. Orange hawkweed Hieracium aurantiacum / IDF & CWH 12. Purple loosestrifeB Lythrum salicaria / CDF & CWH (near Fraser Valley) 13. Scotch broom Cytisus scoparius / CWH & MH 14. Spotted knapweedB Centaurea maculosa / IDF 15. Sulphur cinquefoil Potentilla recta / IDF 16. -

Harmful Non-Indigenous Species in the United States
Harmful Non-Indigenous Species in the United States September 1993 OTA-F-565 NTIS order #PB94-107679 GPO stock #052-003-01347-9 Recommended Citation: U.S. Congress, Office of Technology Assessment, Harmful Non-Indigenous Species in the United States, OTA-F-565 (Washington, DC: U.S. Government Printing Office, September 1993). For Sale by the U.S. Government Printing Office ii Superintendent of Documents, Mail Stop, SSOP. Washington, DC 20402-9328 ISBN O-1 6-042075-X Foreword on-indigenous species (NIS)-----those species found beyond their natural ranges—are part and parcel of the U.S. landscape. Many are highly beneficial. Almost all U.S. crops and domesticated animals, many sport fish and aquiculture species, numerous horticultural plants, and most biologicalN control organisms have origins outside the country. A large number of NIS, however, cause significant economic, environmental, and health damage. These harmful species are the focus of this study. The total number of harmful NIS and their cumulative impacts are creating a growing burden for the country. We cannot completely stop the tide of new harmful introductions. Perfect screening, detection, and control are technically impossible and will remain so for the foreseeable future. Nevertheless, the Federal and State policies designed to protect us from the worst species are not safeguarding our national interests in important areas. These conclusions have a number of policy implications. First, the Nation has no real national policy on harmful introductions; the current system is piecemeal, lacking adequate rigor and comprehensiveness. Second, many Federal and State statutes, regulations, and programs are not keeping pace with new and spreading non-indigenous pests. -

(Heteroptera: Miridae) A
251 CHROMOSOME NUMBERS OF SOME NORTH AMERICAN MIRIDS (HETEROPTERA: MIRIDAE) A. E. AKINGBOHUNGBE Department of Plant Science University of Ife lie-Ife, Nigeria Data are presented on the chromosome numbers (2n) of some eighty species of Miridae. The new information is combined with existing data on some Palearctic and Ethiopian species and discussed. From it, it is suggested that continued reference to 2n - 32A + X + Y as basic mirid karyotype should be avoided and that contrary to earlier suggestions, agmatoploidy rather than poly- ploidy is a more probable mechanism of numerical chromosomal change. Introduction Leston (1957) and Southwood and Leston (1959) gave an account of the available information on chromosome numbers in the Miridae. These works pro- vided the first indication that the subfamilies may show some modalities that might be useful in phylogenetic analysis in the family. Kumar (1971) also gave an ac- count of the karyotype in some six West African cocoa bryocorines. In the present paper, data will be provided on 80 North American mirids, raising to about 131, the number of mirids for which the chromosome numbers are known. Materials and Methods Adult males were collected during the summer of 1970-1972 in Wisconsin and dissected soon after in 0.6% saline solution. The dissected testes were preserved in 3 parts isopropanol: 1 part glacial acetic acid and stored in a referigerator until ready for squashing. Testis squashes were made using Belling's iron-acetocarmine tech- nique as reviewed by Smith (1943) and slides were ringed with either Bennett's zut or Sanford's rubber cement. -

Arthropod Diversity and Conservation in Old-Growth Northwest Forests'
AMER. ZOOL., 33:578-587 (1993) Arthropod Diversity and Conservation in Old-Growth mon et al., 1990; Hz Northwest Forests complex litter layer 1973; Lattin, 1990; JOHN D. LATTIN and other features Systematic Entomology Laboratory, Department of Entomology, Oregon State University, tural diversity of th Corvallis, Oregon 97331-2907 is reflected by the 14 found there (Lawtt SYNOPSIS. Old-growth forests of the Pacific Northwest extend along the 1990; Parsons et a. e coastal region from southern Alaska to northern California and are com- While these old posed largely of conifer rather than hardwood tree species. Many of these ity over time and trees achieve great age (500-1,000 yr). Natural succession that follows product of sever: forest stand destruction normally takes over 100 years to reach the young through successioi mature forest stage. This succession may continue on into old-growth for (Lattin, 1990). Fire centuries. The changing structural complexity of the forest over time, and diseases, are combined with the many different plant species that characterize succes- bances. The prolot sion, results in an array of arthropod habitats. It is estimated that 6,000 a continually char arthropod species may be found in such forests—over 3,400 different ments and habitat species are known from a single 6,400 ha site in Oregon. Our knowledge (Southwood, 1977 of these species is still rudimentary and much additional work is needed Lawton, 1983). throughout this vast region. Many of these species play critical roles in arthropods have lx the dynamics of forest ecosystems. They are important in nutrient cycling, old-growth site, tt as herbivores, as natural predators and parasites of other arthropod spe- mental Forest (HJ cies. -

Entomologische Erhebungen Bei Unterpurkla Und Dirnbach Im Blaurackenbrutgebiet Der Südost-Steiermark
Entomologische Erhebungen bei Unterpurkla und Dirnbach im Blaurackenbrutgebiet der Südost-Steiermark Heuschrecken, Fangschrecken, Wanzen, Tagfalter & Käfer (Saltatoria, Mantodea, Heteroptera, Diurna & Coleoptera) Europaschutzgebiet: „Teile des Südoststeirischen Hügellandes inklusive Grabenlandbäche und Höll“ Im Auftrag von: Verein Lebende Erde im Vulkanland (L.E.i.V.), Bernard Wieser Bearbeitung: Thomas Frieß, Erwin Holzer & Anton Koschuh Graz, im Mai 2012 Entomologische Kartierung 2011 / Dirnbach & Unterpurkla L.E.i.V. Inhaltsverzeichnis 1 Einleitung und Fragestellungen .................................................................................... 3 2 Untersuchte Standorte ................................................................................................... 4 3 Ergebnisse und Diskussion ......................................................................................... 10 3.1 Heuschrecken & Fangschrecken (Saltatoria & Mantodea) ............................... 10 3.2 Wanzen (Heteroptera) .......................................................................................... 16 3.3 Tagfalter (Lepidoptera: Diurna) ........................................................................... 31 3.4 Käfer (Coleoptera) ................................................................................................ 39 4 Literatur .......................................................................................................................... 54 5 Anhang: Rohdatenliste Wanzen ................................................................................. -

Insect Consumption of Seeded Rangeland Herbage in a Selected Area of Diamond Fork Canyon, Utah County, Utah
Utah State University DigitalCommons@USU All Graduate Theses and Dissertations Graduate Studies 5-1976 Insect Consumption of Seeded Rangeland Herbage in a Selected Area of Diamond Fork Canyon, Utah County, Utah Diane M. Bowers Utah State University Follow this and additional works at: https://digitalcommons.usu.edu/etd Part of the Biology Commons Recommended Citation Bowers, Diane M., "Insect Consumption of Seeded Rangeland Herbage in a Selected Area of Diamond Fork Canyon, Utah County, Utah" (1976). All Graduate Theses and Dissertations. 3478. https://digitalcommons.usu.edu/etd/3478 This Thesis is brought to you for free and open access by the Graduate Studies at DigitalCommons@USU. It has been accepted for inclusion in All Graduate Theses and Dissertations by an authorized administrator of DigitalCommons@USU. For more information, please contact [email protected]. INSECT CONSUMPTION OF SEEDED RANGELAND HERBAGE IN A SELECTED AREA OF DIAMOND FORK CANYON, UTAH COUNTY, UTAH by Diane M. Bowers A thesis submitted in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE in Biology UTAH STATE UNIVERSITY Logan, Utah 1976 ACKNOWLEDGMENTS would like to thank my committee for the interest shown, the guidance given, and suggestions made in relation to this study. I am also grateful for their comments in reviewing and editing the manu- script. wish to extend my sincere gratitude to Dr. B. A. Haws, who served as my major professor and to whom am grateful for his personal interest and time given to the project. would also like to thank Dr. Clayton S. Gist and Dr. Cyrus r~. McKell for their suggestions on opera- tional procedures and comments on the rangeland aspect of this study. -

TERRESTRIAL ARTHROPODS 2012-2016 BIOBLITZ VASHON ISLAND List Compiled By: Harsi Parker
COMPLETE LIST OF TERRESTRIAL ARTHROPODS 2012-2016 BIOBLITZ VASHON ISLAND List compiled by: Harsi Parker Number Species name Common name Notes Year Location Taxonomic Order 1 Gammaridae sp. scud 2016 J Amphipoda – Gammaridae 2 Hyalella sp. amphipod 2014, 2016 CH, J Amphipoda – Hyalellidae 3 Acari sp. #1 mite 2012, 2013, 2015, 2016 NP, SH, M, J Arachnida 4 Acari sp. #2 mite 2014 CH Arachnida 5 Opiliones sp. harvestman 2013, 2015 SH, M Arachnida 6 Callobius sp. hacklemesh weaver 2012 NP Arachnida – Amaurobiidae 7 Araneidae sp. orb weaver 2016 J Arachnida – Araneidae 8 Araneus diadematus Cross Orbweaver 2012, 2014 NP, CH Arachnida – Araneidae 9 Clubiona sp. leafcurling sac spider 2012 NP Arachnida – Clubionidae 10 Linyphiinae sp. sheetweb spider tentative ID 2012 NP Arachnida – Linyphiidae 11 Neriene sp. sheetweb spider tentative ID 2014 CH Arachnida – Linyphiidae 12 Pardosa sp. thinlegged wolf spider 2012 NP Arachnida – Lycosidae 13 Philodromus dispar running crab spider 2012 NP Arachnida – Philodromidae 14 Tibellus sp. slender crab spider tentative ID 2014 CH Arachnida – Philodromidae 15 Eris militaris Bronze Jumper tentative ID 2014 CH Arachnida – Salticidae 16 Metaphidippus manni jumping spider tentative ID 2014, 2016 CH, J Arachnida – Salticidae 17 Salticidae sp. #1 jumping spider 2014 CH Arachnida – Salticidae 18 Salticidae sp. #2 jumping spider 2015 M Arachnida – Salticidae 19 Salticus scenicus Zebra Jumper 2013, 2014, 2015 SH, CH, M Arachnida – Salticidae 20 Metellina sp. long-jawed orb weaver 2012 NP Arachnida – Tetragnathidae 21 Tetragnatha sp. long-jawed orb weaver 2013 SH Arachnida – Tetragnathidae 22 Theridiidae sp. cobweb spider 2012 NP Arachnida – Theridiidae 23 Misumena vatia Goldenrod Crab Spider 2013, 2016 SH, J Arachnida – Thomisidae 24 Thomisidae sp. -

Annotated Checklist of the Plant Bug Tribe Mirini (Heteroptera: Miridae: Mirinae) Recorded on the Korean Peninsula, with Descriptions of Three New Species
EUROPEAN JOURNAL OF ENTOMOLOGYENTOMOLOGY ISSN (online): 1802-8829 Eur. J. Entomol. 115: 467–492, 2018 http://www.eje.cz doi: 10.14411/eje.2018.048 ORIGINAL ARTICLE Annotated checklist of the plant bug tribe Mirini (Heteroptera: Miridae: Mirinae) recorded on the Korean Peninsula, with descriptions of three new species MINSUK OH 1, 2, TOMOHIDE YASUNAGA3, RAM KESHARI DUWAL4 and SEUNGHWAN LEE 1, 2, * 1 Laboratory of Insect Biosystematics, Department of Agricultural Biotechnology, Seoul National University, Seoul 08826, Korea; e-mail: [email protected] 2 Research Institute of Agriculture and Life Sciences, Seoul National University, Korea; e-mail: [email protected] 3 Research Associate, Division of Invertebrate Zoology, American Museum of Natural History, New York, NY 10024, USA; e-mail: [email protected] 4 Visiting Scientists, Agriculture and Agri-food Canada, 960 Carling Avenue, Ottawa, Ontario, K1A, 0C6, Canada; e-mail: [email protected] Key words. Heteroptera, Miridae, Mirinae, Mirini, checklist, key, new species, new record, Korean Peninsula Abstract. An annotated checklist of the tribe Mirini (Miridae: Mirinae) recorded on the Korean peninsula is presented. A total of 113 species, including newly described and newly recorded species are recognized. Three new species, Apolygus hwasoonanus Oh, Yasunaga & Lee, sp. n., A. seonheulensis Oh, Yasunaga & Lee, sp. n. and Stenotus penniseticola Oh, Yasunaga & Lee, sp. n., are described. Eight species, Apolygus adustus (Jakovlev, 1876), Charagochilus (Charagochilus) longicornis Reuter, 1885, C. (C.) pallidicollis Zheng, 1990, Pinalitopsis rhodopotnia Yasunaga, Schwartz & Chérot, 2002, Philostephanus tibialis (Lu & Zheng, 1998), Rhabdomiris striatellus (Fabricius, 1794), Yamatolygus insulanus Yasunaga, 1992 and Y. pilosus Yasunaga, 1992 are re- ported for the fi rst time from the Korean peninsula. -

Newsletter No 250 July 2018
Published by RUGBY NATURAL HISTORY SOCIETY www.rugbynaturalhistory.org.uk PRESIDENT – Dr P Reeve Newsletter No 250 July 2018 Contents this edition ~Minibus trip: Rutland Water (book now!) ~News of members ~Summer field visit reports ~ Winter indoor meetings: dates for your diary ~Data protection information ~Current committee members (with contact information) Appendices included: species lists for Grove Hill, Snitterfield Bushes, Dunchurch Meadows, Stockton Cutting and Tasker’s Meadow Photos © Paul Hodges: cowslip carpet; thimble morel; semi-free morel at Grove Hill reserve Minibus trip? Speak up now! Rutland Water. Would you like to travel by minibus to our Rutland Water field visit on Thursday 6 September? Several members requested that we arrange this and David Knapp is willing to do so as long as there is sufficient interest - at least sixteen people would be 1 needed. The cost of a minibus would be £20 per person. The departure/return point would, as usual, be St Mark’s Church car park in Bilton, with additional pick up/drop off points in Long Itchington and Marton. The proposed return visit to Oxford Natural History Museum was cancelled because there were not enough people to make it viable. This is therefore now the FINAL CALL (!) for Rutland Water. If you would be interested in travelling by minibus, please let David know by Wednesday 1 August 2018 and he will then get back to you with further details. Tel. 01788 817346 or e:mail [email protected] News of members Most members will already know that Frank Ollerenshaw died in May. Nine of us attended his funeral, where we learned that he had served in young people’s organisations, as well as being a member both of the society and of Warwickshire Wildlife Trust and having many other interests. -

Feeding Damage by Irbisia Pacifica (Hemiptera: Miridae): Effects of Feeding and Drought on Host Plant Growth
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Entomology Papers from Other Sources Entomology Collections, Miscellaneous 1988 Feeding Damage by Irbisia pacifica (Hemiptera: Miridae): Effects of Feeding and Drought on Host Plant Growth James D. Hansen USDA-ARS, Crops Research Laboratory, Utah State University, Logan, Utah 84322 Robert S. Nowak Department of Range, Wildlife & Forestry, University of Nevada- Reno, Reno, Nevada Follow this and additional works at: https://digitalcommons.unl.edu/entomologyother Part of the Entomology Commons Hansen, James D. and Nowak, Robert S., "Feeding Damage by Irbisia pacifica (Hemiptera: Miridae): Effects of Feeding and Drought on Host Plant Growth" (1988). Entomology Papers from Other Sources. 78. https://digitalcommons.unl.edu/entomologyother/78 This Article is brought to you for free and open access by the Entomology Collections, Miscellaneous at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Entomology Papers from Other Sources by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. Feeding Damage by Irbisia pacifica (Hemiptera: Miridae): Effects of Feeding and Drought on Host Plant Growth 1 JAMES D. HANSEN2,3 AND ROBERT S. NOWAK4 Ann. EntomoL Soc. Am. 81(4): 599-604 (1988) ABSTRACT The interaction of feeding by a grass bug, Ir"bisia pacifica (Uhler), and drought stress on growth of Great Basin wildrye, Leymus cinereus (Scrih. & Merr.) Love, and intermediate wheatgrass, Thinopyrum intermedium (Host) Barkw. & D. R. Dewey, was studied in the greenhouse. At peak production, control plants of Great Basin wildrye had more than twice as much green leaf area per tiller than bug-infested ones, and intermediate wheatgrass controls had three times as much as the bug-infested plants.