Acetification of Porto Wine. a Preliminary Step for Vinegar Making
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Salad Dressing Ratios
Salad Dressing Ratios L E T ' S E X P L O R E H O W T O M A K E S A L A D D R E S S I N G ! Recipes are a wonderful way to teach yourself how to cook, but sometimes you might be missing an ingredient or you might want to try making up your own recipe. The best way to improvise and create your own recipe is by understanding how different factors can change how food works together. For example fat, salt, acid and heat can all drastically change how our food combines and tastes. Fat Salt Acid Heat Remember the TWK@Home activity (Becoming a Sensory Sleuth) where we learned about “sweet”, “salty”, “sour”, and “bitter” and how each of those elements of taste work together in all sorts of things we eat. Chocolate pretzels are a classic example of salty and sweet, and the combination is what makes them taste so good! The best recipes combine the perfect amount of these flavors to create the food we love. Sweet Salty Sour Bitter When making your own salad dressing you want to find a similar balance. But how do you know how much of each ingredient to use without a recipe? Find more TWK@Home activities at tastewisekids.org Written by Kiah Gibian That's where understanding ratios comes in! A ratio is a way to explain how much of one thing there is compared to another thing. For example a good dressing ratio is: 3 parts Oil and 1 part Vinegar or 3 to 1 or Oil 3:1 Vinegar "Parts can be cups, tablespoons or even liters. -

2017 Republic Day Programme Book
29th Annual National Anthem of Canada O Canada, terre de nos aïeux, O Canada! Our home and native land! Ton front est ceint de fleurons glorieux. True patriot love in all thy sons com- Car ton bras sait porter l'épée, mand. Il sait porter la croix. With glowing hearts we see thee rise, Ton histoire est une épopée The True North strong and free! Des plus brillants exploits. From far and wide, O Canada, Et ta valeur, de foi trempée, We stand on guard for thee. Protégera nos foyers et nos droits. God keep our land glorious and free! O Canada, we stand on guard for thee. O Canada, we stand on guard for thee. National Anthem of Mauritius Glory to thee Motherland, O motherland of mine, Thornhill Community Centre Sweet is thy beauty, Sweet is thy fragrance Markham, Ontario Around thee we gather, As one people As one nation Saturday, March 18, 2017 In peace, justice and liberty Beloved Country May God bless thee For ever and ever www.clubmtoronto.com Celebrating 49h Independence Day of Mauritius Celebrating 49th Independence Day of Mauritius Committee Members Jean-Paul HOW Francois LISHINGMAN Edouard LAU MOON LIN Richard LISHINGMAN Oliver LAU MOON LIN Gaetan NG CHEONG TON Jocelyn LEE NAM KWONG Noel SIAO Steve LI CHEE MING Christiane SO TING FONG Jacques LI KAM CHEUNG Stephanie YU FOON MOI Priscilla LI KAM CHEUNG Special thanks to our volunteers and their families: Aurelie Ah-VON Pauline LI CHEE MING Guy CHAN Michelle LISHINGMAN Brigitte HOW Suzanne LISHINGMAN Dean HOW AhYane LOO YONG KEE Bryan KIMSHOOYE Francois LOO YONG KEE Patricia KIMSHOOYE -

Soup in the Glass Vegetable Tempura Counter with Condiment Zucchini
Kitchen Art Brasserie Dinner Menu (A) January to February 2018 (Sunday to Thursday) Selection of Appetizers 6 type of garden lettuce, lolo rossa, lollo bionda, butterhead, Romaine, Frisée, Radicchio, Pasta salad, Thai beef salad, Tauhu sumbat, seafood Caponata, Grated Carrot, Cucumber, capsicum, onion ring, Cherry Tomato, Corn Kernel, & Assorted Lettuce. Assorted Condiments & sesame dressing, cocktail sauce, Italian dressing, sriracha dressing, aioli dressing and 3 type of vinaigrette Soup Chinese soup of the day Italian Breads Counter Assorted Freshly Baked Breads and Rolls with Butter and jams Live stall Lok-Lok Served with 18 condiment and 6 type sauce On ice Rojak buah with condiment In the glass Vegetable Tempura counter with condiment Zucchini, carrot, eggplant, onion ring Asian pizza counter Assorted Asian pizza Stall 1 Noodles station Prawn noodle with condiment Chee Cheong fun and Pau With curry, sweet sauce, spice sauce, sesame and spring onion Stall 2 Pasembor with condiment Stall 3 Live Pasta 3 type of pasta served with fusion sauce, butter milk, kung pow and Provencal sauce Stall 4 Asian Hot Pan Chicken and beef Cabbage, carrot, capsicum, bean sprout, spring onion, local chive, oyster mushroom & Szechuan sauce Stall 5 Chinese stall Yee sang with pomelo, gourmet vegetables salad and special dressing BBQ STREET Mexican marinated barbeque Maple Lamb cutlet, Chicken sausage, caramelize Chicken wings, otak2 Cencaru, kembung,Mix satay with condiment Sauce Hot sauce: Black pepper sauce, and peanut sauce Dipping sauce: mint sauce, -

Hallyu: Riding the Korean Wave
#42 June 2019 Hallyu: Riding the #642 Korean Wave Cross Category Trend Report 1 #642 #472 #47 Introduction The wave of Korean culture, known as Hallyu, is sweeping across the Western world. This cross-category report looks at how this huge cultural trend started. It dives into K-Beauty – one of the big manifestations of the trend, and the Themes, Ingredients and Products driving it. And explores how Korean Culture is transcending other categories like Snacking, Beverages and Alcohol. You’ll also discover how we utilise AI and Social data to surface game-changing insights and scientific trend predictions which help brands understand and action what’s most important in their category, both now and in the future. The information in this report is derived from our Skincare, Beverages, Alcohol and Snacking datasets which are built by analysing millions of publicly available digital consumer conversations from sources including: Twitter, Forums, Blogs, News publications and Reviews. This data is up to date to 31st May 2019. To find out more information or how you can access our datasets and products please visit: blackswan.com #332 #1278 2 BLACKSWANDATA / KOREAN TRENDS REPORT 3 Propelling Korean culture onto the global stage #762 #468 The latest statistics show that thanks to K-Pop 14,000 students are learning Korean Social media and the in the US, compared to only explosion of YouTube 163 two decades earlier brought Korean culture onto the global mainstage The South Korean government through the medium of started championing the K-Pop. exportation of its popular culture with tax breaks and financial backing. -

Crispy Calamari 12
OUTRIGGER CANOE CLUB 2.10.2021 APPETIZERS TRADITIONS Edamame 6 OCC Burger 12 Choice of plain, salted, or spicy garlic House-made Louis sauce, green leaf lettuce, Crispy Calamari 12 tomato, sliced onion 1.00 Sriracha aioli Add cheese Add bacon 2.50 Coconut Shrimp 15 Add avocado 2.50 Mango chili sauce Add jalapeno 1.00 Brussels Sprouts 10 Beyond Burger or Chicken breast can be substituted Tossed with grated parmesan for no additional charge Chicken Wings 15 OCC Mahi Mahi 21 Ranch, celery Lightly battered or flame broiled, steamed rice, sautéed vegetables, lemon caper butter Sliders 12 Vegan Yellow Thai Curry 15 Monterey jack cheese, BBQ sauce, Coconut milk, red and green bell peppers, sweet & tangy bacon jam eggplant, carrot, onion, steamed rice Beef Stew 15 SUSHI & SASHIMI Choice of white or brown steamed rice Spicy Ora King Salmon Roll 14 Hamachi Roll 14 FROM THE CHEF Ebi Tempura Roll 14 Mushroom Bisque 5/8 Spicy Big Eye Tuna Roll 14 California Roll 14 Roasted Bone Marrow 13 Vegetable Roll 12 Parsley pesto, pickled shallot and jalapeno, grilled baguette Ahi Poke Bowl 16 Island style or spicy Chinese Chicken Salad 15 Sashimi Trio 18 Mixed greens, oranges, almonds, carrots, Ahi, Hamachi, King Salmon red onion, fried wun tun pi, sesame vinaigrette SOUP, SALADS & SANDWICHES Spicy Garlic Ahi Roll 14 Marinated garlic ahi, spicy ahi, Portuguese Bean Soup 5/8 fried garlic, scallion Cup or bowl Chicken & Kahuku Papaya 15 Kung Pao Ribeye 32 Curried chicken salad, mango chutney, mixed Choi sum, steamed rice, onion, bell pepper, peanut greens, red -

Great Food, Great Stories from Korea
GREAT FOOD, GREAT STORIE FOOD, GREAT GREAT A Tableau of a Diamond Wedding Anniversary GOVERNMENT PUBLICATIONS This is a picture of an older couple from the 18th century repeating their wedding ceremony in celebration of their 60th anniversary. REGISTRATION NUMBER This painting vividly depicts a tableau in which their children offer up 11-1541000-001295-01 a cup of drink, wishing them health and longevity. The authorship of the painting is unknown, and the painting is currently housed in the National Museum of Korea. Designed to help foreigners understand Korean cuisine more easily and with greater accuracy, our <Korean Menu Guide> contains information on 154 Korean dishes in 10 languages. S <Korean Restaurant Guide 2011-Tokyo> introduces 34 excellent F Korean restaurants in the Greater Tokyo Area. ROM KOREA GREAT FOOD, GREAT STORIES FROM KOREA The Korean Food Foundation is a specialized GREAT FOOD, GREAT STORIES private organization that searches for new This book tells the many stories of Korean food, the rich flavors that have evolved generation dishes and conducts research on Korean cuisine after generation, meal after meal, for over several millennia on the Korean peninsula. in order to introduce Korean food and culinary A single dish usually leads to the creation of another through the expansion of time and space, FROM KOREA culture to the world, and support related making it impossible to count the exact number of dishes in the Korean cuisine. So, for this content development and marketing. <Korean Restaurant Guide 2011-Western Europe> (5 volumes in total) book, we have only included a selection of a hundred or so of the most representative. -

A Carrot by Any Other Name… New Looks at Your Old Co-Op by Shannon Szymkowiak, Promotions & Education Manager & WFC Owner
20 WINTER 2013 GARBANZO GAZETTE back 40 special items in your produce department by Claire Musech, Produce Buyer/ Receiver & WFC Owner You may have already noticed the for flowers, and you receive and 15% have a gorgeous assortment of Poin- bright, new addition to your Co-op—it’s discount if you are a WFC Owner! Place settias, Norfolk Pines, Frosty Ferns, fresh flower bouquets and plants! them at the Customer Service Counter, Rosemary Cones, Golden Cypress and I have had the pleasure of working with or with anyone in the Produce Depart- Silver Bush set to arrive on December Koehler and Dramm Wholesale Florist ment or you can just call it in. 13th. Guarantee your first choice by put- in Minneapolis to bring you beautiful ting in a special order with the Produce We are also working on sourcing local and affordable cut flower bouquets, Department today as quantities are and regional fall mums from the Amish arrangements and plants. We have limited. and an assortment of local hanging been able to provide a unique selection baskets in the spring. This is a small Add a distinguishing touch to your of house plants ranging from traditional but exciting step towards our store goal Holiday gathering by arriving with ones like Ivy and Pathos to the unusual of having 20 percent of all products a Co-op made fruit and vegetable tray varieties like Venus fly trap and sold be LOCAL by the year 2020! or fruit and gift basket. We are happy Cypress. Whether you are choosing to take special requests within 48 hours a gift to brighten someone’s day Everyone loves flowers for the Holidays. -

Screening of Acetic Acid Producing Microorganisms from Decomposed Fruits for Vinegar Production
Advances in Microbiology, 2015, 5, 291-297 Published Online May 2015 in SciRes. http://www.scirp.org/journal/aim http://dx.doi.org/10.4236/aim.2015.55028 Screening of Acetic Acid Producing Microorganisms from Decomposed Fruits for Vinegar Production Farzana Diba1, Fahmida Alam1,2, Ali Azam Talukder1* 1Department of Microbiology, Jahangirnagar University, Dhaka, Bangladesh 2Human Genome Centre, School of Medical Sciences, Universiti Sains Malaysia, Kubang Kerian, Malaysia Email: *[email protected], [email protected] Received 11 February 2015; accepted 5 May 2015; published 7 May 2015 Copyright © 2015 by authors and Scientific Research Publishing Inc. This work is licensed under the Creative Commons Attribution International License (CC BY). http://creativecommons.org/licenses/by/4.0/ Abstract Acetic acid bacteria capable of growing at 30˚C - 37˚C were collected from various decomposed fruits available in Bangladeshi local markets in order to assess their suitability for vinegar pro- duction. Initially, 42 microorganisms were isolated from decomposed fruits like grapes, mangoes, pineapples, oranges, safeda etc. during summer when temperature reaches up to 37˚C. Then their growths were checked in YPG medium containing various ethanol concentrations at different time point at 37˚C. From the preliminary screening, 15 Gram negative bacterial isolates have produced halos or yellow zone around the colonies on YPG agar plate at 37˚C which indicated acetic acid production capability by those bacteria. Furthermore, acetic acid production rates were deter- mined by titration method and about 3 - 6.9 gm/100ml acetic acid were estimated by using 4% ethanol at 37˚C by shaking culture for 3 days. -
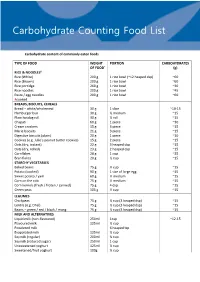
Carbohydrate Counting List
Tr45 Carbohydrate Counting Food List Carbohydrate content of commonly eaten foods TYPE OF FOOD WEIGHT PORTION CARBOHYDRATES OF FOOD* (g) RICE & NOODLES# Rice (White) 200 g 1 rice bowl (~12 heaped dsp) ~60 Rice (Brown) 200 g 1 rice bowl ~60 Rice porridge 260 g 1 rice bowl ~30 Rice noodles 200 g 1 rice bowl ~45 Pasta / egg noodles 200 g 1 rice bowl ~60 #cooked BREADS, BISCUITS, CEREALS Bread – white/wholemeal 30 g 1 slice ~10-15 Hamburger bun 30 g ½ medium ~15 Plain hotdog roll 30 g ½ roll ~15 Chapati 60 g 1 piece ~30 Cream crackers 15 g 3 piece ~15 Marie biscuits 21 g 3 piece ~15 Digestive biscuits (plain) 20 g 1 piece ~10 Cookies (e.g. Julie’s peanut butter cookies) 15 g 2 piece ~15 Oats (dry, instant) 22 g 3 heaped dsp ~15 Oats (dry, rolled) 23 g 2 heaped dsp ~15 Cornflakes 28 g 1 cup ~25 Bran flakes 20 g ½ cup ~15 STARCHY VEGETABLES Baked beans 75 g ⅓ cup ~15 Potato (cooked) 90 g 1 size of large egg ~15 Sweet potato / yam 60 g ½ medium ~15 Corn on the cob 75 g ½ medium ~15 Corn kernels (fresh / frozen / canned) 75 g 4 dsp ~15 Green peas 105 g ½ cup ~15 LEGUMES Chickpeas 75 g ½ cup (3 heaped dsp) ~15 Lentils (e.g. Dhal) 75 g ½ cup (3 heaped dsp) ~15 Beans – green / red / black / mung 75 g ½ cup (3 heaped dsp) ~15 MILK AND ALTERNATIVES Liquid milk (non-flavoured) 250ml 1cup ~12-15 Flavoured milk 125ml ½ cup Powdered milk 6 heaped tsp Evaporated milk 125ml ½ cup Soymilk (regular) 200ml ¾ cup Soymilk (reduced sugar) 250ml 1 cup Unsweetened yoghurt 125ml ½ cup Sweetened/fruit yoghurt 100g ⅓ cup TYPE OF FOOD WEIGHT PORTION CARBOHYDRATES OF -

An Overview on Types, Medicinal Uses and Production of Vinegar
The Pharma Innovation Journal 2019; 8(6): 1083-1087 ISSN (E): 2277- 7695 ISSN (P): 2349-8242 NAAS Rating: 5.03 An overview on types, medicinal uses and production of TPI 2019; 8(6): 1083-1087 © 2019 TPI vinegar www.thepharmajournal.com Received: 10-04-2019 Accepted: 12-05-2019 Avinash A Sankpal Avinash A Sankpal Department of Pharmaceutics, Abstract Satara College of Pharmacy, Vinegar is the fermented product which consisting about 5-20% of acetic acid, prepared by fermentation Satara. Maharashtra, India of alcohol with the help of Acecobactor species. Vinegar is the food additive it is used in ketchup, salad dressing and in pickle. It is also used as food preservatives. The use of vinegar as a medicine is firstly carried out by Hippocrates. He used vinegar for the treatment of wound healing. Different types of Vinegar are present in the world. The different possible medicinal uses of Vinegar are reviewed in the present review. Vinegar is used as Antidiabetic, Antimicrobial, Antioxidant, Antitumor, Antiobesity, it reduces Cholesterol level, it maintains different Brain functions and it is also used in Injuries. In present Article we are reviewed all previous work which are carried out on the Vinegar including Method of preparation, Characterization of Vinegar and uses of Vinegar etc. The Vinegar is prepared with the help of different methods like artificial method and natural fermentation method etc. The characterization of Vinegar is mainly carried out with the help of following tests pH, Titratable acidity, Specific gravity etc. Keywords: Vinegar, types, uses of vinegar, fermentation, characterization 1. Introduction Vinegar is prepared by different methods and from various raw materials. -

Developing the Optimal Vinaigrette Dressing for Managing Blood Glucose
Developing the Optimal Vinaigrette Dressing for Managing Blood Glucose Concentrations by Amber K. Bonsall A Thesis Presented in Partial Fulfillment of the Requirements for the Degree Master of Science Approved August 2016 by the Graduate Supervisory Committee: Carol Johnston, Chair Sandra Mayol-Kreiser Christy Lespron ARIZONA STATE UNIVERSITY May 2017 ABSTRACT Background: Acetic acid in vinegar has demonstrated antiglycemic effects in previous studies; however, the mechanism is unknown. Objective: To determine whether acetic acid dissociates in the addition of sodium chloride and describe a flavorful vinaigrette that maintains the functional properties of acetic acid. Design: Phase I - Ten healthy subjects (23-40 years) taste tested five homemade vinaigrette and five commercial dressings. Perceived saltiness, sweetness, tartness, and overall tasted were scored using a modified labeled affective magnitude scale. Each dressing was tested three times for pH with a calibrated meter. Phase II – Randomized crossover trial testing six dressings against a control dressing two groups of nine healthy adult subjects (18-52 years). Height, weight and calculated body mass index (BMI) were performed at baseline. Subjects participated in four test sessions each, at least seven days apart. After a 10-hour fast, participants consumed 38g of the test drink, followed by a bagel meal. Capillary blood glucose was obtained at fasting, and every 30 minutes over a 2-hour period the test meal. Results: Dressing pH reduced as sodium content increased. In the intervention trials, no significant differences were observed between groups (p >0.05). The greatest reduction in postprandial glycemia (~21%) was observed in the dressing containing 200 mg of sodium. -

Determination of Volatile Compounds in Fresh and Fermented Nipa Sap (Nypa Fruticans) Using Static Headspace Gas Chromatography-Mass Spectrometry (GC-MS)
International Food Research Journal 20(1): 369-376 (2013) Journal homepage: http://www.ifrj.upm.edu.my Determination of volatile compounds in fresh and fermented Nipa sap (Nypa fruticans) using static headspace gas chromatography-mass spectrometry (GC-MS) 1Nur Aimi, R., 1,2*Abu Bakar, F. and 1Dzulkifly, M. H. 1Halal Products Research Institute, Universiti Putra Malaysia, 43400 Serdang, Malaysia 2Department of Food Science, Faculty of Food Science and Technology, Universiti Putra Malaysia, 43400 Serdang, Malaysia Article history Abstract Received: 5 April 2012 Nipa sap or air nira is a sweet natural beverage obtained from a type of palm tree, Nypa fruticans. Received in revised form: It is readily and spontaneously fermented resulting in the development of alcoholic fermentation 24 May 2012 products. Objective of this study is to determine the volatile compounds (VOCs) responsible Accepted: 25 May 2012 for the aroma in fresh and fermented nipa sap. The sap was left for natural fermentation at 30ºC for 63 days. VOCs of the sap were analysed using static headspace gas chromatography-mass spectrometry (GC-MS). Fresh nipa sap contained ethanol (83.43%), diacetyl (0.59%), and esters (15.97%). Fermented nipa sap contained alcohols (91.16 – 98.29%), esters (1.18 – 8.14%), acetoin (0.02 – 0.7%), diacetyl (0.04 – 0.06%), and acetic acid (0.13 – 0.68%). Concentration of ethanol in fresh nipa sap increased from 0.11% (v/v) to 6.63% (v/v) during the fermentation, Keywords and slightly decreased to 5.73% (v/v) at day 63. No higher alcohols were detected in the fresh nipa sap.