Phase Diagrams
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Thermodynamics
TREATISE ON THERMODYNAMICS BY DR. MAX PLANCK PROFESSOR OF THEORETICAL PHYSICS IN THE UNIVERSITY OF BERLIN TRANSLATED WITH THE AUTHOR'S SANCTION BY ALEXANDER OGG, M.A., B.Sc., PH.D., F.INST.P. PROFESSOR OF PHYSICS, UNIVERSITY OF CAPETOWN, SOUTH AFRICA THIRD EDITION TRANSLATED FROM THE SEVENTH GERMAN EDITION DOVER PUBLICATIONS, INC. FROM THE PREFACE TO THE FIRST EDITION. THE oft-repeated requests either to publish my collected papers on Thermodynamics, or to work them up into a comprehensive treatise, first suggested the writing of this book. Although the first plan would have been the simpler, especially as I found no occasion to make any important changes in the line of thought of my original papers, yet I decided to rewrite the whole subject-matter, with the inten- tion of giving at greater length, and with more detail, certain general considerations and demonstrations too concisely expressed in these papers. My chief reason, however, was that an opportunity was thus offered of presenting the entire field of Thermodynamics from a uniform point of view. This, to be sure, deprives the work of the character of an original contribution to science, and stamps it rather as an introductory text-book on Thermodynamics for students who have taken elementary courses in Physics and Chemistry, and are familiar with the elements of the Differential and Integral Calculus. The numerical values in the examples, which have been worked as applications of the theory, have, almost all of them, been taken from the original papers; only a few, that have been determined by frequent measurement, have been " taken from the tables in Kohlrausch's Leitfaden der prak- tischen Physik." It should be emphasized, however, that the numbers used, notwithstanding the care taken, have not vii x PREFACE. -

Low Temperature Soldering Using Sn-Bi Alloys
LOW TEMPERATURE SOLDERING USING SN-BI ALLOYS Morgana Ribas, Ph.D., Anil Kumar, Divya Kosuri, Raghu R. Rangaraju, Pritha Choudhury, Ph.D., Suresh Telu, Ph.D., Siuli Sarkar, Ph.D. Alpha Assembly Solutions, Alpha Assembly Solutions India R&D Centre Bangalore, KA, India [email protected] ABSTRACT package substrate and PCB [2-4]. This represents a severe Low temperature solder alloys are preferred for the limitation on using the latest generation of ultra-thin assembly of temperature-sensitive components and microprocessors. Use of low temperature solders can substrates. The alloys in this category are required to reflow significantly reduce such warpage, but available Sn-Bi between 170 and 200oC soldering temperatures. Lower solders do not match Sn-Ag-Cu drop shock performance [5- soldering temperatures result in lower thermal stresses and 6]. Besides these pressing technical requirements, finding a defects, such as warping during assembly, and permit use of low temperature solder alloy that can replace alloys such as lower cost substrates. Sn-Bi alloys have lower melting Sn-3Ag-0.5Cu solder can result in considerable hard dollar temperatures, but some of its performance drawbacks can be savings from reduced energy cost and noteworthy reduction seen as deterrent for its use in electronics devices. Here we in carbon emissions [7]. show that non-eutectic Sn-Bi alloys can be used to improve these properties and further align them with the electronics In previous works [8-11] we have showed how the use of industry specific needs. The physical properties and drop micro-additives in eutectic Sn-Bi alloys results in significant shock performance of various alloys are evaluated, and their improvement of its thermo-mechanical properties. -

Recent Progress on Deep Eutectic Solvents in Biocatalysis Pei Xu1,2, Gao‑Wei Zheng3, Min‑Hua Zong1,2, Ning Li1 and Wen‑Yong Lou1,2*
Xu et al. Bioresour. Bioprocess. (2017) 4:34 DOI 10.1186/s40643-017-0165-5 REVIEW Open Access Recent progress on deep eutectic solvents in biocatalysis Pei Xu1,2, Gao‑Wei Zheng3, Min‑Hua Zong1,2, Ning Li1 and Wen‑Yong Lou1,2* Abstract Deep eutectic solvents (DESs) are eutectic mixtures of salts and hydrogen bond donors with melting points low enough to be used as solvents. DESs have proved to be a good alternative to traditional organic solvents and ionic liq‑ uids (ILs) in many biocatalytic processes. Apart from the benign characteristics similar to those of ILs (e.g., low volatil‑ ity, low infammability and low melting point), DESs have their unique merits of easy preparation and low cost owing to their renewable and available raw materials. To better apply such solvents in green and sustainable chemistry, this review frstly describes some basic properties, mainly the toxicity and biodegradability of DESs. Secondly, it presents several valuable applications of DES as solvent/co-solvent in biocatalytic reactions, such as lipase-catalyzed transester‑ ifcation and ester hydrolysis reactions. The roles, serving as extractive reagent for an enzymatic product and pretreat‑ ment solvent of enzymatic biomass hydrolysis, are also discussed. Further understanding how DESs afect biocatalytic reaction will facilitate the design of novel solvents and contribute to the discovery of new reactions in these solvents. Keywords: Deep eutectic solvents, Biocatalysis, Catalysts, Biodegradability, Infuence Introduction (1998), one concept is “Safer Solvents and Auxiliaries”. Biocatalysis, defned as reactions catalyzed by bio- Solvents represent a permanent challenge for green and catalysts such as isolated enzymes and whole cells, has sustainable chemistry due to their vast majority of mass experienced signifcant progress in the felds of either used in catalytic processes (Anastas and Eghbali 2010). -

The Basics of Soldering
TECH SOLUTION: SOLDER The Basics of Soldering SUmmARY BY Chris Nash, INDIUM CORPORATION IN In this article, I will present a basic overview of soldering for those Soldering uses a filler metal and, in who are new to the world of most cases, an appropriate flux. The soldering and for those who could filler metals are typically alloys (al- though there are some pure metal sol- use a refresher. I will discuss the ders) that have liquidus temperatures below 350°C. Elemental metals com- definition of soldering, the basics monly alloyed in the filler metals or of metallurgy, how to choose the solders are tin, lead, antimony, bis- muth, indium, gold, silver, cadmium, proper alloy, the purpose of a zinc, and copper. By far, the most flux, soldering temperatures, and common solders are based on tin. Fluxes often contain rosin, acids (or- typical heating sources for soldering ganic or mineral), and/or halides, de- operations. pending on the desired flux strength. These ingredients reduce the oxides on the solder and mating pieces. hroughout history, as society has evolved, so has the need for bond- Basic Solder Metallurgy ing metals to metals. Whether the As heat is gradually applied to sol- need for bonding metals is mechani- der, the temperature rises until the Tcal, electrical, or thermal, it can be accom- alloy’s solidus point is reached. The plished by using solder. solidus point is the highest temper- ature at which an alloy is completely Metallurgical Bonding Processes solid. At temperatures just above Attachment of one metal to another can solidus, the solder is a mixture of be accomplished in three ways: welding, liquid and solid phases (analogous brazing, and soldering. -

Robust High-Temperature Heat Exchangers (Topic 2A Gen 3 CSP Project; DE-EE0008369)
Robust High-Temperature Heat Exchangers (Topic 2A Gen 3 CSP Project; DE-EE0008369) Illustrations of: (left) porous WC preform plates, (middle) dense-wall ZrC/W plates with horizontal channels and vertical vias. (Right) Backscattered electron image of the dense microstructure of a ZrC/W cermet. Team: Ken H. Sandhage1 (PI), Kevin P. Trumble1 (Co-PI), Asegun Henry2 (Co-PI), Aaron Wildberger3 (Co-PI) 1School of Materials Engineering, Purdue University, W. Lafayette, IN 2Department of Mechanical Engineering, Massachusetts Institute of Technology, Cambridge, MA 3Vacuum Process Engineering, Inc., Sacramento, CA Concentrated Solar Power Tower “Concentrating Solar Power Gen3 Demonstration Roadmap,” M. Mehos, C. Turchi, J. Vidal, M. Wagner, Z. Ma, C. Ho, W. Kolb, C. Andraka, A. Kruizenga, Technical Report NREL/TP-5500-67464, National Renewable Energy Laboratory, 2017 Concentrated Solar Power Tower Heat Exchanger “Concentrating Solar Power Gen3 Demonstration Roadmap,” M. Mehos, C. Turchi, J. Vidal, M. Wagner, Z. Ma, C. Ho, W. Kolb, C. Andraka, A. Kruizenga, Technical Report NREL/TP-5500-67464, National Renewable Energy Laboratory, 2017 State of the Art: Metal Alloy Printed Circuit HEXs Current Technology: • Printed Circuit HEXs: patterned etching of metallic alloy plates, then diffusion bonding • Metal alloy mechanical properties degrade significantly above 600oC D. Southall, S.J. Dewson, Proc. ICAPP '10, San Diego, CA, 2010; R. Le Pierres, et al., Proc. SCO2 Power Cycle Symposium 2011, Boulder, CO, 2011; D. Southall, et al., Proc. ICAPP '08, Anaheim, -
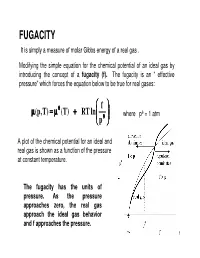
FUGACITY It Is Simply a Measure of Molar Gibbs Energy of a Real Gas
FUGACITY It is simply a measure of molar Gibbs energy of a real gas . Modifying the simple equation for the chemical potential of an ideal gas by introducing the concept of a fugacity (f). The fugacity is an “ effective pressure” which forces the equation below to be true for real gases: θθθ f µµµ ,p( T) === µµµ (T) +++ RT ln where pθ = 1 atm pθθθ A plot of the chemical potential for an ideal and real gas is shown as a function of the pressure at constant temperature. The fugacity has the units of pressure. As the pressure approaches zero, the real gas approach the ideal gas behavior and f approaches the pressure. 1 If fugacity is an “effective pressure” i.e, the pressure that gives the right value for the chemical potential of a real gas. So, the only way we can get a value for it and hence for µµµ is from the gas pressure. Thus we must find the relation between the effective pressure f and the measured pressure p. let f = φ p φ is defined as the fugacity coefficient. φφφ is the “fudge factor” that modifies the actual measured pressure to give the true chemical potential of the real gas. By introducing φ we have just put off finding f directly. Thus, now we have to find φ. Substituting for φφφ in the above equation gives: p µ=µ+(p,T)θ (T) RT ln + RT ln φ=µ (ideal gas) + RT ln φ pθ µµµ(p,T) −−− µµµ(ideal gas ) === RT ln φφφ This equation shows that the difference in chemical potential between the real and ideal gas lies in the term RT ln φφφ.φ This is the term due to molecular interaction effects. -

MICROALLOYED Sn-Cu Pb-FREE SOLDER for HIGH TEMPERATURE APPLICATIONS
As originally published in the SMTA Proceedings. MICROALLOYED Sn-Cu Pb-FREE SOLDER FOR HIGH TEMPERATURE APPLICATIONS Keith Howell1, Keith Sweatman1, Motonori Miyaoka1, Takatoshi Nishimura1, Xuan Quy Tran2, Stuart McDonald2, and Kazuhiro Nogita2 1 Nihon Superior Co., Ltd., Osaka, Japan 2 The University of Queensland, Brisbane, Australia [email protected] require any of the materials or substances listed in Annex II (of the Directive) ABSTRACT • is scientifically or technical impracticable While the search continues for replacements for the • the reliability of the substitutes is not ensured highmelting-point, high-Pb solders on which the • the total negative environmental, health and electronics industry has depended for joints that maintain consumer safety impacts caused by substitution are their integrity at high operating temperatures, an likely to outweigh the total environmental, health investigation has been made into the feasibility of using a and safety benefits thereof.” hypereutectic Sn-7Cu in this application. While its solidus temperature remains at 227°C the microstructure, which A recast of the Directive issued in June 2011 includes the has been substantially modified by stabilization and grain statement that for such exemptions “the maximum validity refining of the primary Cu6Sn5 by microalloying additions period which may be renewed shall… be 5 years from 21 July of Ni and Al, makes it possible for this alloy to maintain its 2011”. The inference from this statement is that there is an integrity and adequate strength even after long term expectation that alternatives to the use of solders with a lead exposure to temperatures up to 150°C. In this paper the content of 85% or more will be found before 21st July 2016. -

Lecture 15: 11.02.05 Phase Changes and Phase Diagrams of Single- Component Materials
3.012 Fundamentals of Materials Science Fall 2005 Lecture 15: 11.02.05 Phase changes and phase diagrams of single- component materials Figure removed for copyright reasons. Source: Abstract of Wang, Xiaofei, Sandro Scandolo, and Roberto Car. "Carbon Phase Diagram from Ab Initio Molecular Dynamics." Physical Review Letters 95 (2005): 185701. Today: LAST TIME .........................................................................................................................................................................................2� BEHAVIOR OF THE CHEMICAL POTENTIAL/MOLAR FREE ENERGY IN SINGLE-COMPONENT MATERIALS........................................4� The free energy at phase transitions...........................................................................................................................................4� PHASES AND PHASE DIAGRAMS SINGLE-COMPONENT MATERIALS .................................................................................................6� Phases of single-component materials .......................................................................................................................................6� Phase diagrams of single-component materials ........................................................................................................................6� The Gibbs Phase Rule..................................................................................................................................................................7� Constraints on the shape of -

A Study of the Equilibrium Diagrams of the Systems, Benzene-Toluene and Benzene-Ethylbenzene
Loyola University Chicago Loyola eCommons Master's Theses Theses and Dissertations 1940 A Study of the Equilibrium Diagrams of the Systems, Benzene- Toluene and Benzene-Ethylbenzene John B. Mullen Loyola University Chicago Follow this and additional works at: https://ecommons.luc.edu/luc_theses Part of the Chemistry Commons Recommended Citation Mullen, John B., "A Study of the Equilibrium Diagrams of the Systems, Benzene-Toluene and Benzene- Ethylbenzene" (1940). Master's Theses. 299. https://ecommons.luc.edu/luc_theses/299 This Thesis is brought to you for free and open access by the Theses and Dissertations at Loyola eCommons. It has been accepted for inclusion in Master's Theses by an authorized administrator of Loyola eCommons. For more information, please contact [email protected]. This work is licensed under a Creative Commons Attribution-Noncommercial-No Derivative Works 3.0 License. Copyright © 1940 John B. Mullen A S'l'IJDY OF THE EQUTI.IBRIUM DIAGRAMS OF THE SYSTEMS, BENZENE-TOLUENE AND BENZENE-~ENZENE By John B. Mullen Presented in partial :f'u.J.tilment at the requirements :f'or the degree o:f' Master o:f' Science, Loyola University, 1940. T.ABLE OF CONTENTS Page Acknowledgment 1i Vita iii Introduction 1 I. Review of Literature 2 II. Apparatus and its Calibration 3 III. Procedure am Technique 6 IV. Observations on the System Benzene-Toluene 9 V. Observations on the System Benzene-Ethylbenzene 19 VI. Equilibrium Diagrams of the Systems 30 Recommendations for Future Work 34 Bibliography 35 ACKNOWLEOOMENT The au1hor wishes to acknowledge with thanks the invaluable assistance, suggestions, and cooperation offered by Dr. -

Phase Transitions in Multicomponent Systems
Physics 127b: Statistical Mechanics Phase Transitions in Multicomponent Systems The Gibbs Phase Rule Consider a system with n components (different types of molecules) with r phases in equilibrium. The state of each phase is defined by P,T and then (n − 1) concentration variables in each phase. The phase equilibrium at given P,T is defined by the equality of n chemical potentials between the r phases. Thus there are n(r − 1) constraints on (n − 1)r + 2 variables. This gives the Gibbs phase rule for the number of degrees of freedom f f = 2 + n − r A Simple Model of a Binary Mixture Consider a condensed phase (liquid or solid). As an estimate of the coordination number (number of nearest neighbors) think of a cubic arrangement in d dimensions giving a coordination number 2d. Suppose there are a total of N molecules, with fraction xB of type B and xA = 1 − xB of type A. In the mixture we assume a completely random arrangement of A and B. We just consider “bond” contributions to the internal energy U, given by εAA for A − A nearest neighbors, εBB for B − B nearest neighbors, and εAB for A − B nearest neighbors. We neglect other contributions to the internal energy (or suppose them unchanged between phases, etc.). Simple counting gives the internal energy of the mixture 2 2 U = Nd(xAεAA + 2xAxBεAB + xBεBB) = Nd{εAA(1 − xB) + εBBxB + [εAB − (εAA + εBB)/2]2xB(1 − xB)} The first two terms in the second expression are just the internal energy of the unmixed A and B, and so the second term, depending on εmix = εAB − (εAA + εBB)/2 can be though of as the energy of mixing. -

Introduction to Phase Diagrams*
ASM Handbook, Volume 3, Alloy Phase Diagrams Copyright # 2016 ASM InternationalW H. Okamoto, M.E. Schlesinger and E.M. Mueller, editors All rights reserved asminternational.org Introduction to Phase Diagrams* IN MATERIALS SCIENCE, a phase is a a system with varying composition of two com- Nevertheless, phase diagrams are instrumental physically homogeneous state of matter with a ponents. While other extensive and intensive in predicting phase transformations and their given chemical composition and arrangement properties influence the phase structure, materi- resulting microstructures. True equilibrium is, of atoms. The simplest examples are the three als scientists typically hold these properties con- of course, rarely attained by metals and alloys states of matter (solid, liquid, or gas) of a pure stant for practical ease of use and interpretation. in the course of ordinary manufacture and appli- element. The solid, liquid, and gas states of a Phase diagrams are usually constructed with a cation. Rates of heating and cooling are usually pure element obviously have the same chemical constant pressure of one atmosphere. too fast, times of heat treatment too short, and composition, but each phase is obviously distinct Phase diagrams are useful graphical representa- phase changes too sluggish for the ultimate equi- physically due to differences in the bonding and tions that show the phases in equilibrium present librium state to be reached. However, any change arrangement of atoms. in the system at various specified compositions, that does occur must constitute an adjustment Some pure elements (such as iron and tita- temperatures, and pressures. It should be recog- toward equilibrium. Hence, the direction of nium) are also allotropic, which means that the nized that phase diagrams represent equilibrium change can be ascertained from the phase dia- crystal structure of the solid phase changes with conditions for an alloy, which means that very gram, and a wealth of experience is available to temperature and pressure. -

Neutron Star Cooling and Discuss the Main Issue: What We Can Learn About the Internal Structure of Neutron Stars by Confronting Theory and Observation
To appear in Ann. Rev. Astron. Astrophys. 2004 Neutron Star Cooling D. G. Yakovlev 1 and C. J. Pethick 2 1Ioffe Physical Technical Institute, Politekhnicheskaya 26, 194021 St.-Petersburg, Russia, e-mail: [email protected]ffe.ru 2NORDITA, The Nordic Institute for Theoretical Physics, Blegdamsvej 17, DK– 2100 Copenhagen Ø, Denmark, e-mail: [email protected] Abstract Observation of cooling neutron stars can potentially provide information about the states of matter at supernuclear densities. We review physical properties important for cooling such as neutrino emission processes and superfluidity in the stellar interior, surface envelopes of light elements due to accretion of matter and strong surface magnetic fields. The neutrino pro- cesses include the modified Urca process, and the direct Urca process for nucleons and exotic states of matter such as a pion condensate, kaon con- densate, or quark matter. The dependence of theoretical cooling curves on physical input and observations of thermal radiation from isolated neutron stars are described. The comparison of observation and theory leads to a unified interpretation in terms of three characteristic types of neutron stars: high-mass stars which cool primarily by some version of the direct Urca process; low-mass stars, which cool via slower processes; and medium-mass stars, which have an intermediate behavior. The related problem of thermal states of transiently accreting neutron stars with deep crustal burning of accreted matter is discussed in connection with observations of soft X-ray transients. arXiv:astro-ph/0402143v1 6 Feb 2004 1 INTRODUCTION Neutron stars are the most compact stars in the Universe. They have masses M ∼ 1.4 M⊙ and radii R ∼ 10 km, and they contain matter at supernuclear densities in their cores.