Cataract and Optic Disk Drusen in a Patient with Glycogenosis and Di George Syndrome: Clinical and Molecular Report D
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Isyte: Integrated Systems Tool for Eye Gene Discovery
Lens iSyTE: Integrated Systems Tool for Eye Gene Discovery Salil A. Lachke,1,2,3,4 Joshua W. K. Ho,1,4,5 Gregory V. Kryukov,1,4,6 Daniel J. O’Connell,1 Anton Aboukhalil,1,7 Martha L. Bulyk,1,8,9 Peter J. Park,1,5,10 and Richard L. Maas1 PURPOSE. To facilitate the identification of genes associated ther investigation. Extension of this approach to other ocular with cataract and other ocular defects, the authors developed tissue components will facilitate eye disease gene discovery. and validated a computational tool termed iSyTE (integrated (Invest Ophthalmol Vis Sci. 2012;53:1617–1627) DOI: Systems Tool for Eye gene discovery; http://bioinformatics. 10.1167/iovs.11-8839 udel.edu/Research/iSyTE). iSyTE uses a mouse embryonic lens gene expression data set as a bioinformatics filter to select candidate genes from human or mouse genomic regions impli- ven with the advent of high-throughput sequencing, the cated in disease and to prioritize them for further mutational Ediscovery of genes associated with congenital birth defects and functional analyses. such as eye defects remains a challenge. We sought to develop METHODS. Microarray gene expression profiles were obtained a straightforward experimental approach that could facilitate for microdissected embryonic mouse lens at three key devel- the identification of candidate genes for developmental disor- opmental time points in the transition from the embryonic day ders, and, as proof-of-principle, we chose defects involving the (E)10.5 stage of lens placode invagination to E12.5 lens primary ocular lens. Opacification of the lens results in cataract, a leading cause of blindness that affects 77 million persons and fiber cell differentiation. -

A New Mutation in BFSP2 (G1091A) Causes Autosomal Dominant Congenital Lamellar Cataracts
Molecular Vision 2008; 14:1906-1911 <http://www.molvis.org/molvis/v14/a226> © 2008 Molecular Vision Received 17 August 2008 | Accepted 18 October 2008 | Published 24 October 2008 A new mutation in BFSP2 (G1091A) causes autosomal dominant congenital lamellar cataracts Xu Ma,1,2,3 Fei-Feng Li,1,2 Shu-Zhen Wang,4 Chang Gao,1,2 Meng Zhang,2 Si-Quan Zhu4 (The first three authors contributed equally to this work.) 1Graduate School, Peking Union Medical College, Beijing, China; 2Department of Genetics, National Research Institute for Family Planning, Beijing, China; 3WHO Collaborative Center for Research in Human Reproduction, Beijing, China; 4Beijing Tongren Eye Center, Capital Medical University, Beijing, China Purpose: We sought to identify the genetic defect in a four-generation Chinese family with autosomal dominant congenital lamellar cataracts and demonstrate the functional analysis with biosoftware of a candidate gene in the family. Methods: Family history data were recorded. Clinical and ophthalmologic examinations were performed on family members. All the members were genotyped with microsatellite markers at loci considered to be associated with cataracts. Two-point LOD scores were calculated by using the Linkage Software after genotyping. A mutation was detected by using gene-specific primers in direct sequencing. Wild type and mutant proteins were analyzed with Online Bio-Software. Results: Affected members of this family had lamellar cataracts. Linkage analysis was obtained at markers D3S2322 (LOD score [Z]=7.22, recombination fraction [θ]=0.0) and D3S1541 (Z=5.42, θ=0.0). Haplotype analysis indicated that the cataract gene was closely linked to these two markers. -

Localization of the Lens Intermediate Filament Switch by Imaging Mass Spectrometry
bioRxiv preprint doi: https://doi.org/10.1101/2020.04.21.053793; this version posted April 23, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Localization of the Lens Intermediate Filament Switch by Imaging Mass Spectrometry Zhen Wang, Daniel J. Ryan, and Kevin L Schey* Department of Biochemistry, Vanderbilt University, Nashville, TN 37232 * To whom correspondence should be addressed Current address: Mass Spectrometry Research Center Vanderbilt University 465 21st Ave. So., Suite 9160 MRB III Nashville, TN 37232-8575 E-mail: [email protected] Phone: 615.936.6861 Fax: 615.343.8372 bioRxiv preprint doi: https://doi.org/10.1101/2020.04.21.053793; this version posted April 23, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Abstract Imaging mass spectrometry (IMS) enables targeted and untargeted visualization of the spatial localization of molecules in tissues with great specificity. The lens is a unique tissue that contains fiber cells corresponding to various stages of differentiation that are packed in a highly spatial order. The application of IMS to lens tissue localizes molecular features that are spatially related to the fiber cell organization. Such spatially resolved molecular information assists our understanding of lens structure and physiology; however, protein IMS studies are typically limited to abundant, soluble, low molecular weight proteins. In this study, a method was developed for imaging low solubility cytoskeletal proteins in the lens; a tissue that is filled with high concentrations of soluble crystallins. -

Variants in PAX6, PITX3 and HSF4 Causing Autosomal Dominant Congenital Cataracts ✉ ✉ Vanita Berry 1,2 , Alex Ionides2, Nikolas Pontikos 1,2, Anthony T
www.nature.com/eye ARTICLE OPEN Variants in PAX6, PITX3 and HSF4 causing autosomal dominant congenital cataracts ✉ ✉ Vanita Berry 1,2 , Alex Ionides2, Nikolas Pontikos 1,2, Anthony T. Moore2, Roy A. Quinlan3 and Michel Michaelides 1,2 © Crown 2021 BACKGROUND: Lens development is orchestrated by transcription factors. Disease-causing variants in transcription factors and their developmental target genes are associated with congenital cataracts and other eye anomalies. METHODS: Using whole exome sequencing, we identified disease-causing variants in two large British families and one isolated case with autosomal dominant congenital cataract. Bioinformatics analysis confirmed these disease-causing mutations as rare or novel variants, with a moderate to damaging pathogenicity score, with testing for segregation within the families using direct Sanger sequencing. RESULTS: Family A had a missense variant (c.184 G>A; p.V62M) in PAX6 and affected individuals presented with nuclear cataract. Family B had a frameshift variant (c.470–477dup; p.A160R*) in PITX3 that was also associated with nuclear cataract. A recurrent missense variant in HSF4 (c.341 T>C; p.L114P) was associated with congenital cataract in a single isolated case. CONCLUSIONS: We have therefore identified novel variants in PAX6 and PITX3 that cause autosomal dominant congenital cataract. Eye; https://doi.org/10.1038/s41433-021-01711-x INTRODUCTION consistent with early developmental effects as would be Cataract the opacification of the eye lens is the most common, but anticipated for PAX6 and PITX3 transcription factors. Recently, treatable cause of blindness in the world (https://www.who.int/ we have found two novel mutations in the transcription factors publications-detail/world-report-on-vision). -

A Computational Approach for Defining a Signature of Β-Cell Golgi Stress in Diabetes Mellitus
Page 1 of 781 Diabetes A Computational Approach for Defining a Signature of β-Cell Golgi Stress in Diabetes Mellitus Robert N. Bone1,6,7, Olufunmilola Oyebamiji2, Sayali Talware2, Sharmila Selvaraj2, Preethi Krishnan3,6, Farooq Syed1,6,7, Huanmei Wu2, Carmella Evans-Molina 1,3,4,5,6,7,8* Departments of 1Pediatrics, 3Medicine, 4Anatomy, Cell Biology & Physiology, 5Biochemistry & Molecular Biology, the 6Center for Diabetes & Metabolic Diseases, and the 7Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN 46202; 2Department of BioHealth Informatics, Indiana University-Purdue University Indianapolis, Indianapolis, IN, 46202; 8Roudebush VA Medical Center, Indianapolis, IN 46202. *Corresponding Author(s): Carmella Evans-Molina, MD, PhD ([email protected]) Indiana University School of Medicine, 635 Barnhill Drive, MS 2031A, Indianapolis, IN 46202, Telephone: (317) 274-4145, Fax (317) 274-4107 Running Title: Golgi Stress Response in Diabetes Word Count: 4358 Number of Figures: 6 Keywords: Golgi apparatus stress, Islets, β cell, Type 1 diabetes, Type 2 diabetes 1 Diabetes Publish Ahead of Print, published online August 20, 2020 Diabetes Page 2 of 781 ABSTRACT The Golgi apparatus (GA) is an important site of insulin processing and granule maturation, but whether GA organelle dysfunction and GA stress are present in the diabetic β-cell has not been tested. We utilized an informatics-based approach to develop a transcriptional signature of β-cell GA stress using existing RNA sequencing and microarray datasets generated using human islets from donors with diabetes and islets where type 1(T1D) and type 2 diabetes (T2D) had been modeled ex vivo. To narrow our results to GA-specific genes, we applied a filter set of 1,030 genes accepted as GA associated. -

Ck1δ Over-Expressing Mice Display ADHD-Like Behaviors, Frontostriatal Neuronal Abnormalities and Altered Expressions of ADHD-Candidate Genes
Molecular Psychiatry (2020) 25:3322–3336 https://doi.org/10.1038/s41380-018-0233-z ARTICLE CK1δ over-expressing mice display ADHD-like behaviors, frontostriatal neuronal abnormalities and altered expressions of ADHD-candidate genes 1 1 1 2 1 1 1 Mingming Zhou ● Jodi Gresack ● Jia Cheng ● Kunihiro Uryu ● Lars Brichta ● Paul Greengard ● Marc Flajolet Received: 8 November 2017 / Revised: 4 July 2018 / Accepted: 18 July 2018 / Published online: 19 October 2018 © Springer Nature Limited 2018 Abstract The cognitive mechanisms underlying attention-deficit hyperactivity disorder (ADHD), a highly heritable disorder with an array of candidate genes and unclear genetic architecture, remain poorly understood. We previously demonstrated that mice overexpressing CK1δ (CK1δ OE) in the forebrain show hyperactivity and ADHD-like pharmacological responses to D- amphetamine. Here, we demonstrate that CK1δ OE mice exhibit impaired visual attention and a lack of D-amphetamine- induced place preference, indicating a disruption of the dopamine-dependent reward pathway. We also demonstrate the presence of abnormalities in the frontostriatal circuitry, differences in synaptic ultra-structures by electron microscopy, as 1234567890();,: 1234567890();,: well as electrophysiological perturbations of both glutamatergic and GABAergic transmission, as observed by altered frequency and amplitude of mEPSCs and mIPSCs. Furthermore, gene expression profiling by next-generation sequencing alone, or in combination with bacTRAP technology to study specifically Drd1a versus Drd2 medium spiny neurons, revealed that developmental CK1δ OE alters transcriptional homeostasis in the striatum, including specific alterations in Drd1a versus Drd2 neurons. These results led us to perform a fine molecular characterization of targeted gene networks and pathway analysis. Importantly, a large fraction of 92 genes identified by GWAS studies as associated with ADHD in humans are significantly altered in our mouse model. -

Common Variants in SOX-2 and Congenital Cataract Genes Contribute to Age-Related Nuclear Cataract
ARTICLE https://doi.org/10.1038/s42003-020-01421-2 OPEN Common variants in SOX-2 and congenital cataract genes contribute to age-related nuclear cataract Ekaterina Yonova-Doing et al.# 1234567890():,; Nuclear cataract is the most common type of age-related cataract and a leading cause of blindness worldwide. Age-related nuclear cataract is heritable (h2 = 0.48), but little is known about specific genetic factors underlying this condition. Here we report findings from the largest to date multi-ethnic meta-analysis of genome-wide association studies (discovery cohort N = 14,151 and replication N = 5299) of the International Cataract Genetics Consortium. We confirmed the known genetic association of CRYAA (rs7278468, P = 2.8 × 10−16) with nuclear cataract and identified five new loci associated with this dis- ease: SOX2-OT (rs9842371, P = 1.7 × 10−19), TMPRSS5 (rs4936279, P = 2.5 × 10−10), LINC01412 (rs16823886, P = 1.3 × 10−9), GLTSCR1 (rs1005911, P = 9.8 × 10−9), and COMMD1 (rs62149908, P = 1.2 × 10−8). The results suggest a strong link of age-related nuclear cat- aract with congenital cataract and eye development genes, and the importance of common genetic variants in maintaining crystalline lens integrity in the aging eye. #A list of authors and their affiliations appears at the end of the paper. COMMUNICATIONS BIOLOGY | (2020) 3:755 | https://doi.org/10.1038/s42003-020-01421-2 | www.nature.com/commsbio 1 ARTICLE COMMUNICATIONS BIOLOGY | https://doi.org/10.1038/s42003-020-01421-2 ge-related cataract is the leading cause of blindness, structure (meta-analysis genomic inflation factor λ = 1.009, accounting for more than one-third of blindness Supplementary Table 4 and Supplementary Fig. -

Proteome Profiling of Developing Murine Lens Through Mass
Lens Proteome Profiling of Developing Murine Lens Through Mass Spectrometry Shahid Y. Khan,1 Muhammad Ali,1 Firoz Kabir,1 Santosh Renuse,2 Chan Hyun Na,2 C. Conover Talbot Jr,3 Sean F. Hackett,1 and S. Amer Riazuddin1 1The Wilmer Eye Institute, Johns Hopkins University School of Medicine, Baltimore, Maryland, United States 2Department of Biological Chemistry, Johns Hopkins University School of Medicine, Baltimore, Maryland, United States 3Institute for Basic Biomedical Sciences, Johns Hopkins University School of Medicine, Baltimore, Maryland, United States Correspondence: S. Amer Riazuddin, PURPOSE. We previously completed a comprehensive profile of the mouse lens transcriptome. The Wilmer Eye Institute, Johns Here, we investigate the proteome of the mouse lens through mass spectrometry–based Hopkins University School of Medi- protein sequencing at the same embryonic and postnatal time points. cine, 600 N. Wolfe Street; Maumenee 840, Baltimore, MD 21287, USA; METHODS. We extracted mouse lenses at embryonic day 15 (E15) and 18 (E18) and postnatal [email protected]. day 0 (P0), 3 (P3), 6 (P6), and 9 (P9). The lenses from each time point were preserved in three Submitted: February 2, 2017 distinct pools to serve as biological replicates for each developmental stage. The total cellular Accepted: October 13, 2017 protein was extracted from the lens, digested with trypsin, and labeled with isobaric tandem mass tags (TMT) for three independent TMT experiments. Citation: Khan SY, Ali M, Kabir F, et al. Proteome profiling of developing mu- RESULTS. A total of 5404 proteins were identified in the mouse ocular lens in at least one rine lens through mass spectrometry. -

Signal Transduction in L-DOPA-Induced Dyskinesia: from Receptor Sensitization to Abnormal… Et Al
Journal of Neural Transmission https://doi.org/10.1007/s00702-018-1847-7 NEUROLOGY AND PRECLINICAL NEUROLOGICAL STUDIES - REVIEW ARTICLE Signal transduction in L‑DOPA‑induced dyskinesia: from receptor sensitization to abnormal gene expression Giada Spigolon1 · Gilberto Fisone1 Received: 1 November 2017 / Accepted: 23 January 2018 © The Author(s) 2018. This article is an open access publication Abstract A large number of signaling abnormalities have been implicated in the emergence and expression of L-DOPA-induced dyskinesia (LID). The primary cause for many of these changes is the development of sensitization at dopamine receptors located on striatal projection neurons (SPN). This initial priming, which is particularly evident at the level of dopamine D1 receptors (D1R), can be viewed as a homeostatic response to dopamine depletion and is further exacerbated by chronic administration of L-DOPA, through a variety of mechanisms afecting various components of the G-protein-coupled receptor machinery. Sensitization of dopamine receptors in combination with pulsatile administration of L-DOPA leads to intermit- tent and coordinated hyperactivation of signal transduction cascades, ultimately resulting in long-term modifcations of gene expression and protein synthesis. A detailed mapping of these pathological changes and of their involvement in LID has been produced during the last decade. According to this emerging picture, activation of sensitized D1R results in the stimulation of cAMP-dependent protein kinase and of the dopamine- and cAMP-regulated phosphoprotein of 32 kDa. This, in turn, activates the extracellular signal-regulated kinases 1 and 2 (ERK), leading to chromatin remodeling and aberrant gene transcription. Dysregulated ERK results also in the stimulation of the mammalian target of rapamycin complex 1, which promotes protein synthesis. -

Avian Binocularity and Adaptation to Nocturnal Environments: Genomic Insights Froma Highly Derived Visual Phenotype Rui Borges Universidade Do Porto - Portugal
Nova Southeastern University NSUWorks Biology Faculty Articles Department of Biological Sciences 8-22-2019 Avian Binocularity and Adaptation to Nocturnal Environments: Genomic Insights froma Highly Derived Visual Phenotype Rui Borges Universidade do Porto - Portugal Joao Fonseca Universidade do Porto - Portugal Cidalia Gomes Universidade do Porto - Portugal Warren E. Johnson Smithsonian Institution Stephen James O'Brien St. Petersburg State University - Russia; Nova Southeastern University, [email protected] See next page for additional authors Follow this and additional works at: https://nsuworks.nova.edu/cnso_bio_facarticles Part of the Biology Commons NSUWorks Citation Borges, Rui; Joao Fonseca; Cidalia Gomes; Warren E. Johnson; Stephen James O'Brien; Guojie Zhang; M. Thomas P. Gilbert; Erich D. Jarvis; and Agostinho Antunes. 2019. "Avian Binocularity and Adaptation to Nocturnal Environments: Genomic Insights froma Highly Derived Visual Phenotype." Genome Biology and Evolution 11, (8): 2244-2255. doi:10.1093/gbe/evz111. This Article is brought to you for free and open access by the Department of Biological Sciences at NSUWorks. It has been accepted for inclusion in Biology Faculty Articles by an authorized administrator of NSUWorks. For more information, please contact [email protected]. Authors Rui Borges, Joao Fonseca, Cidalia Gomes, Warren E. Johnson, Stephen James O'Brien, Guojie Zhang, M. Thomas P. Gilbert, Erich D. Jarvis, and Agostinho Antunes This article is available at NSUWorks: https://nsuworks.nova.edu/cnso_bio_facarticles/982 GBE Avian Binocularity and Adaptation to Nocturnal Environments: Genomic Insights from a Highly Derived Visual Downloaded from https://academic.oup.com/gbe/article-abstract/11/8/2244/5544263 by Nova Southeastern University/HPD Library user on 16 September 2019 Phenotype Rui Borges1,2,Joao~ Fonseca1,Cidalia Gomes1, Warren E. -
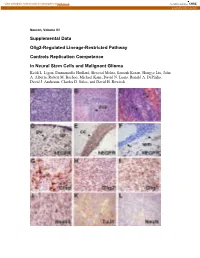
Supplemental Data Olig2-Regulated Lineage-Restricted Pathway Controls Replication Competence in Neural Stem Cells and Malignant
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Caltech Authors Neuron, Volume 53 Supplemental Data Olig2-Regulated Lineage-Restricted Pathway Controls Replication Competence in Neural Stem Cells and Malignant Glioma Keith L. Ligon, Emmanuelle Huillard, Shwetal Mehta, Santosh Kesari, Hongye Liu, John A. Alberta, Robert M. Bachoo, Michael Kane, David N. Louis, Ronald A. DePinho, David J. Anderson, Charles D. Stiles, and David H. Rowitch Figure S1. Olig1/2+/- Ink4a/Arf-/-EGFRvIII Gliomas Express Characteristic Morphologic and Immunophenotypic Features of Human Malignant Gliomas (A) Tumors exhibit dense cellularity and atypia. (B) Characteristic infiltration of host SVZ stem cell niche. (C) Although occasional tumors exhibited pseudopalisading necrosis (n, arrowhead) and hemorrhage (h, arrowhead) similar to human GBM (Astrocytoma WHO Grade IV), most tumors lacked these features. (D) IHC for hEGFR highlights hallmark feature of human gliomas, including perivascular (pv) and subpial (sp) accumulation of tumor cells, (E) striking white matter tropism of tumor cells in corpus callosum (cc), and (F) distant single cell infiltration of cerebellar white matter (wm, arrows). (G-J) Immunohistochemical markers characteristic of human tumors (Gfap, Olig2, Olig1, Nestin) are present. (K and L) Weak staining for the early neuronal marker TuJ1 and absence of the differentiated neuronal marker, NeuN similar to human glioma and consistent with heterogeneous lines of partial differentiation. Figure S2. Olig1/2+/- Ink4a/Arf-/-EGFRvIII Neurospheres Are Multipotent and Olig1/2-/- Ink4a/Arf-/-EGFRvIII Neurospheres Are Bipotent Olig1/2+/- or Olig1/2-/- Ink4a/Arf-/-EGFRvIII neurospheres were allowed to differentiate for 6 days in medium without EGF. -

Filensin (BFSP1) (NM 001195) Human Tagged ORF Clone Product Data
OriGene Technologies, Inc. 9620 Medical Center Drive, Ste 200 Rockville, MD 20850, US Phone: +1-888-267-4436 [email protected] EU: [email protected] CN: [email protected] Product datasheet for RC213412 Filensin (BFSP1) (NM_001195) Human Tagged ORF Clone Product data: Product Type: Expression Plasmids Product Name: Filensin (BFSP1) (NM_001195) Human Tagged ORF Clone Tag: Myc-DDK Symbol: BFSP1 Synonyms: CP94; CP115; CTRCT33; LIFL-H Vector: pCMV6-Entry (PS100001) E. coli Selection: Kanamycin (25 ug/mL) Cell Selection: Neomycin This product is to be used for laboratory only. Not for diagnostic or therapeutic use. View online » ©2021 OriGene Technologies, Inc., 9620 Medical Center Drive, Ste 200, Rockville, MD 20850, US 1 / 6 Filensin (BFSP1) (NM_001195) Human Tagged ORF Clone – RC213412 ORF Nucleotide >RC213412 representing NM_001195 Sequence: Red=Cloning site Blue=ORF Green=Tags(s) TTTTGTAATACGACTCACTATAGGGCGGCCGGGAATTCGTCGACTGGATCCGGTACCGAGGAGATCTGCC GCCGCGATCGCC ATGTACCGGCGCAGCTACGTCTTCCAGACCCGCAAGGAGCAGTACGAGCACGCCGACGAGGCTTCGCGCG CCGCCGAGCCCGAGCGCCCGGCCGACGAGGGCTGGGCTGGGGCAACGAGCCTGGCGGCGCTGCAGGGGCT CGGCGAGCGCGTGGCCGCCCACGTCCAGCGGGCCCGCGCCCTCGAGCAGCGCCATGCCGGGCTCCGGAGG CAGCTGGATGCCTTCCAGCGCCTGGGCGAGCTGGCCGGGCCCGAGGACGCCCTCGCCCGCCAAGTCGAGA GCAACCGCCAGCGCGTCCGGGACCTGGAGGCCGAGCGCGCCCGGCTGGAGCGCCAGGGCACCGAGGCGCA GCGCGCGCTCGACGAGTTCCGAAGCAAGTATGAAAATGAGTGCGAATGTCAACTCCTGCTAAAAGAAATG CTTGAACGGCTTAACAAGGAAGCTGATGAAGCCTTGCTGCATAACCTACGCCTTCAGCTGGAAGCCCAAT TTCTGCAAGATGATATCAGTGCGGCAAAGGACAGGCACAAGAAGAATCTTCTGGAAGTTCAGACCTATAT CAGCATCCTGCAGCAGATCATCCACACCACTCCTCCAGCATCCATTGTGACGAGTGGGATGAGGGAGGAG