Ecophysiology and Reproductive Biology of Cultivated Cacti
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Aspects of House Finch Breeding Biology in Hawaii
ASPECTS OF HOUSE FINCH BREEDING BIOLOGY IN HAWAII CHARLES VAN RIPER III Bent (1968) summarized information avail- Puu Laau, is the last remaining major mamane-naio able on the breeding biology of the House forest in Hawaii. Finch ( Curpodacus mexicanus). Although The stippled areas of figure 1 represent a broad spectrum of the forest types on the island of Hawaii; this species has been studied quite extensively included are native, introduced, and mixed stands of in its North American home range, little atten- vegetation. Areas 2, 3, and 5 are dry forest regions tion has been paid to it in Hawaii. Grinnell with annual rainfall of 76 cm or less; Puu Laau (2) (1911) reported on different color patterns of has mean annual rainfall of 50 cm, Puu Waawaa (3) 64 cm, and Puu Lehua (5) has 76 cm. The Kohala the House Finch in Hawaii, and Richardson Mountain complex ( 1) has a mean annual rainfall of and Bowles (1964) mentioned that on 23 June 229 cm, Puu 00 (4) has 483 cm, and the Kulani- 1960 they found a nestling that had fallen from Mauna Loa complex (6) has 317 cm. its nest on Kauai. On Mauna Kea, Berger Birds were mist-netted, color-banded, and released (1972) found House Finch nests with eggs from 1971 through 1973. Nest and tree heights were taken with a clinometer when it was impractical to as early as 6 April (1968) and as late as 17 use a tape measure. Nests and eggs were measured July (1967). Eleven nests were built on hori- with calipers and weighed on a sensitive spring bal- zontal branches of mamane (Sophora chryso- ance. -

Department of the Interior Fish and Wildlife Service
Thursday, February 27, 2003 Part II Department of the Interior Fish and Wildlife Service 50 CFR Part 17 Endangered and Threatened Wildlife and Plants; Final Designation or Nondesignation of Critical Habitat for 95 Plant Species From the Islands of Kauai and Niihau, HI; Final Rule VerDate Jan<31>2003 13:12 Feb 26, 2003 Jkt 200001 PO 00000 Frm 00001 Fmt 4717 Sfmt 4717 E:\FR\FM\27FER2.SGM 27FER2 9116 Federal Register / Vol. 68, No. 39 / Thursday, February 27, 2003 / Rules and Regulations DEPARTMENT OF THE INTERIOR units designated for the 83 species. This FOR FURTHER INFORMATION CONTACT: Paul critical habitat designation requires the Henson, Field Supervisor, Pacific Fish and Wildlife Service Service to consult under section 7 of the Islands Office at the above address Act with regard to actions carried out, (telephone 808/541–3441; facsimile 50 CFR Part 17 funded, or authorized by a Federal 808/541–3470). agency. Section 4 of the Act requires us SUPPLEMENTARY INFORMATION: RIN 1018–AG71 to consider economic and other relevant impacts when specifying any particular Background Endangered and Threatened Wildlife area as critical habitat. This rule also and Plants; Final Designation or In the Lists of Endangered and determines that designating critical Nondesignation of Critical Habitat for Threatened Plants (50 CFR 17.12), there habitat would not be prudent for seven 95 Plant Species From the Islands of are 95 plant species that, at the time of species. We solicited data and Kauai and Niihau, HI listing, were reported from the islands comments from the public on all aspects of Kauai and/or Niihau (Table 1). -
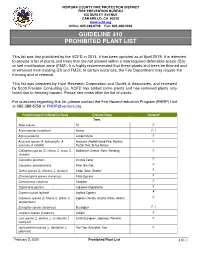
Guideline 410 Prohibited Plant List
VENTURA COUNTY FIRE PROTECTION DISTRICT FIRE PREVENTION BUREAU 165 DURLEY AVENUE CAMARILLO, CA 93010 www.vcfd.org Office: 805-389-9738 Fax: 805-388-4356 GUIDELINE 410 PROHIBITED PLANT LIST This list was first published by the VCFD in 2014. It has been updated as of April 2019. It is intended to provide a list of plants and trees that are not allowed within a new required defensible space (DS) or fuel modification zone (FMZ). It is highly recommended that these plants and trees be thinned and or removed from existing DS and FMZs. In certain instances, the Fire Department may require the thinning and or removal. This list was prepared by Hunt Research Corporation and Dudek & Associates, and reviewed by Scott Franklin Consulting Co, VCFD has added some plants and has removed plants only listed due to freezing hazard. Please see notes after the list of plants. For questions regarding this list, please contact the Fire Hazard reduction Program (FHRP) Unit at 085-389-9759 or [email protected] Prohibited plant list:Botanical Name Common Name Comment* Trees Abies species Fir F Acacia species (numerous) Acacia F, I Agonis juniperina Juniper Myrtle F Araucaria species (A. heterophylla, A. Araucaria (Norfolk Island Pine, Monkey F araucana, A. bidwillii) Puzzle Tree, Bunya Bunya) Callistemon species (C. citrinus, C. rosea, C. Bottlebrush (Lemon, Rose, Weeping) F viminalis) Calocedrus decurrens Incense Cedar F Casuarina cunninghamiana River She-Oak F Cedrus species (C. atlantica, C. deodara) Cedar (Atlas, Deodar) F Chamaecyparis species (numerous) False Cypress F Cinnamomum camphora Camphor F Cryptomeria japonica Japanese Cryptomeria F Cupressocyparis leylandii Leyland Cypress F Cupressus species (C. -

Non-Native Trees Provide Habitat for Native Hawaiian Forest Birds
NON-NATIVE TREES PROVIDE HABITAT FOR NATIVE HAWAIIAN FOREST BIRDS By Peter J. Motyka A Thesis Submitted in Partial Fulfillment Of the Requirements for the Degree of Master of Science In Biology Northern Arizona University December 2016 Approved: Jeffrey T. Foster, Ph.D., Co-chair Tad C. Theimer, Ph. D., Co-chair Carol L. Chambers, Ph. D. ABSTRACT NON-NATIVE TREES PROVIDE HABITAT FOR NATIVE HAWAIIAN FOREST BIRDS PETER J. MOTYKA On the Hawaiian island of Maui, native forest birds occupy an area dominated by non- native plants that offers refuge from climate-limited diseases that threaten the birds’ persistence. This study documented the status of the bird populations and their ecology in this novel habitat. Using point-transect distance sampling, I surveyed for birds over five periods in 2013-2014 at 123 stations across the 20 km² Kula Forest Reserve (KFR). I documented abundance and densities for four native bird species: Maui ‘alauahio (Paroreomyza montana), ʻiʻiwi (Drepanis coccinea), ʻapapane (Himatione sanguinea), and Hawaiʻi ʻamakihi, (Chlorodrepanis virens), and three introduced bird species: Japanese white-eye (Zosterops japonicas), red-billed leiothrix (Leiothrix lutea), and house finch (Haemorhous mexicanus). I found that 1) native forest birds were as abundant as non-natives, 2) densities of native forest birds in the KFR were similar to those found in native forests, 3) native forest birds showed varying dependence on the structure of the habitats, with ʻiʻiwi and ‘alauahio densities 20 and 30 times greater in forest than in scrub, 4) Maui ‘alauahio foraged most often in non-native cape wattle, eucalyptus, and tropical ash, and nested most often in non-native Monterey cypress, Monterey pine, and eucalyptus. -

List 01 Hawaiian Names 01 Plants
V\.{). 3 v BOTANICAL BULLETIN NO.2 JUNE. 1913 TERRITORY OF HAWAII BOARD OF AGRICULTURE AND FORESTRY List 01 Hawaiian Names 01 Plants BY JOSEPH F. ROCK Consulting Botanist, Board of Agriculture and Forestry HONOLULU: HAWAIIAN GAZETTE CO., LTD. 1913 ALPHABETICAL LIST OF HAWAIIAN NAMES OF PLANTS. The following list of Hawaiian plant-names has been compiled from various sources. Hillebrand in his valuable Flora of the Hawaiian Islands has given many Hawaiian names, especially of the more common species; these are incorporated in this list with a few corrections. Nearly all Hawaiian plant-names found in this list and not in Hillebrand's Flora were secured from Mr. Francis Gay of the Island of Kauai, an old resident in this Terri tory and well acquainted with its plants from a layman's stand point. It was the writer's privilege to camp with Mr. Gay in the mountains of Kauai collecting botanical material; for almost every species he could give the native name, which he had se cured in the early days from old and reliable natives. Mr. Gay had made spatter prints of many of the native plants in a large record book with their names and uses, as well as their symbolic meaning when occurring in mele (songs) or olioli (chants), at tached to them. For all this information the writer is indebted mainly to Mr. Francis Gay and also to Mr. Augustus F. Knudsen of the same Island. The writer also secured Hawaiian names from old na tives and Kahunas (priests) in the various islands of the group. -

Akeake (Dodonaea Viscosa) Number 26
Akeake (Dodonaea viscosa) Number 26 DESCRIPTION Akeake is easily recognised by its distinctive bright green long thin wavy leaves, flaking bark and its papery winged seed capsules. It is a hardy, coastal shrub or small tree. In the right conditions, it will grow to about 10m high with a spreading crown and a trunk of around 30cm in diameter. The leaves are alternate, thin, light green with smooth margins and raised veins. The bark is reddish brown and peels off in long, thin strips. Akeake flowers are non-descript. They form in panicles at the end of branches from spring through to mid-summer. The fruit, however, are very visible as a capsule with two to four pale yellow wings which turn brown when dry and ripe. There are usually one or two seeds per capsule. DISTRIBUTION Akeake is native to New Zealand and grows throughout the North Island and northern parts of the South Island. It has also been found on the Chatham Islands, where it may have been introduced. It is also widely distributed through the Southern Hemisphere, with different subspecies and varieties in Australia, Southeast Asia, Africa and Latin CONSERVATION America. Akeake is not a nationally threatened or endangered plant. It is recognised as being Akeake naturally occurs in coastal and regionally distinctive, as it is generally lowland zones, favouring areas of open uncommon in the Taranaki region. forest or scrub. It prefers drier sites such as coastal river valleys, steep hillsides and THREATS cliffs and stabilised dunes. It is drought- Trampling and browsing by domestic tolerant and will handle light frosts. -

Dodonaea Viscosa Jacq. SAPINDACEAE Synonym: Dodonaea Angustifolia
Trees and Shrubs of the Maldives 83 Dodonaea viscosa Jacq. SAPINDACEAE Synonym: Dodonaea angustifolia . Common names: Giant bush hop, sand olive Dhivehi name: Kudhi-ruuvaali Status: Common in some of the islands in the southern group; rare in the northern islands. Description: A fast-growing small tree or shrub that grows to 1 to 3 m in height but is capable of reaching 8 m. Bark is dark grey coloured, fissured and peeling. Branchlets are rusty red in colour, sometimes narrowly winged or ridged. Branches exudate resin. Leaves are simple, papery with very short petiole or subsessile. Leaf shape varies from lanceolate to linear spooniform and secretes gummy exudate on both the surfaces and thus always appearing shiny. Leaf margin is entire or inconspicuously wavy and the leaf tip is pointed or round. Inflorescence is terminal or axillary and densely flowered. Flowers are very small, borne on long and slender flower stalks. Sepals are four in number, greenish-yellow in colour; petals are absent. Fruit is a capsule and two to three winged. In mature fruits, wings are coral red in colour. Seeds are small, black and lens like in shape. Each chamber of the fruit contains one to two seeds. Uses: D. viscosa is very effective in sand dune fixation and controlling coastal erosion since its roots are excellent soil binders. It is also used to reclaim marshes. It is grown as an ornamental plant for its shiny foliage and pink-red winged fruit. Poles are useful in fencing. Timber is hard and durable. In the Maldives, tree nails are prepared from the timber, which are used for boat building. -

Crop Ecology, Cultivation and Uses of Cactus Pear
CROP ECOLOGY, CULTIVATION AND USES OF CACTUS PEAR Advance draft prepared for the IX INTERNATIONAL CONGRESS ON CACTUS PEAR AND COCHINEAL CAM crops for a hotter and drier world Coquimbo, Chile, 26-30 March 2017 CROP ECOLOGY, CULTIVATION AND USES OF CACTUS PEAR Editorial team Prof. Paolo Inglese, Università degli Studi di Palermo, Italy; General Coordinator Of the Cactusnet Dr. Candelario Mondragon, INIFAP, Mexico Dr. Ali Nefzaoui, ICARDA, Tunisia Prof. Carmen Sáenz, Universidad de Chile, Chile Coordination team Makiko Taguchi, FAO Harinder Makkar, FAO Mounir Louhaichi, ICARDA Editorial support Ruth Duffy Book design and layout Davide Moretti, Art&Design − Rome Published by the Food and Agriculture Organization of the United Nations and the International Center for Agricultural Research in the Dry Areas Rome, 2017 The designations employed and the FAO encourages the use, reproduction and presentation of material in this information dissemination of material in this information product do not imply the expression of any product. Except where otherwise indicated, opinion whatsoever on the part of the Food material may be copied, downloaded and Agriculture Organization of the United and printed for private study, research Nations (FAO), or of the International Center and teaching purposes, or for use in non- for Agricultural Research in the Dry Areas commercial products or services, provided (ICARDA) concerning the legal or development that appropriate acknowledgement of FAO status of any country, territory, city or area as the source and copyright holder is given or of its authorities, or concerning the and that FAO’s endorsement of users’ views, delimitation of its frontiers or boundaries. -

The Biology, Host Range, Parasites, and Hyperparasites of Koa Seed Insects in Hawaii: a Review
Vol. 24, Nos. 2 & 3, October 15,1983 317 The Biology, Host Range, Parasites, and Hyperparasites of Koa Seed Insects in Hawaii: a Review JOHN D. STEIN1 ABSTRACT The biology and host range of koa seed insects, their parasites, and hyperparasites in Hawaii are reviewed. The information reported may be applicable to other native or introduced legumes because of the wide hosthnst rangeranopt ofrtf a fewfi»w ofnf thetht* insects.inc^Wc Koa, Acacia koa Gray, is considered the most valuable native timber species in Hawaii. Pure stands of koa cover approximately 7.5 thousand hectares (18.6 thousand acres) with an additional 172.4 thousand hectares (426 thousand acres) of koa-ohia mixture in the native forest ecosystems within the State. Selective logging has reduced the quality of koa to less desirable commercial grade trees. Since 1978, the Hawaii State Department of Land and Natural Resources has been replanting sites where koa once grew. The emphasis on reforestation of this high value hardwood has stimulated research by the Forest Service, U.S. Department of Agriculture, to select and propagate genetically superior trees. Progeny from these trees will then be used to establish viable seed orchards. Insects present a potentially serious threat to koa seed production. In a recent survey, I found that up to 86% of the seed was destroyed by insects, and three insects were responsible for 93% of the damage (Stein 1983). This review discusses the biology and host range of the koa seed insects, and lists their parasites. Previously published biological data for these insects were augmented with information from the Bernice P. -

Exjobb 2003-7
Department of Silviculture Graduate thesis 2005-17 The Role of Indigenous Knowledge in Forest Management A Case Study from Masol and Sook Division, West Pokot, Kenya Ida Wigrup Illustration: Pernilla Lonhage Graduate thesis in Forest Management Supervisor: Kenneth Sahlén Examiner: Arne Albrektson Department of Silviculture Swedish University of Agricultural Sciences Umeå 2005 The Role of Indigenous Knowledge in Forest Management A Case Study from Masol and Sook Division, West Pokot, Kenya Ida Wigrup Abstract Indigenous knowledge (IK) concerning forestry is, in many societies around the world, in danger of being lost, since western science has lately been controlling the development of forest management practices to such a large extent. It is therefore of the greatest importance to record and assess such IK before it becomes extinct. The objective of the study was to do this for the traditional knowledge that the local population in Masol and Sook Division, Kenya, possessed regarding the use, reproductive processes and deliberate dispersal of trees. Furthermore, to examine the current balance between the people and the forest resource and to put the result in relation to the role of future forest development interventions in the area. The result of the study is based on 40 interviews with both women and men. The lifestyle in Masol Division is still traditional and they are pastoralists, the lifestyle in Sook Division has been undergoing changes lately and they are now more settled and are cultivating the land to greater extent than in the past. The study revealed that the IK was limited to meeting the direct needs which the population in the study area had experienced. -

SGAP Cairns Home Page
Newsletter SGAP Cairns March 2014 Society for Growing Australian Plants, Cairns Branch this issue Most people are familiar with the concept of photosynthesis – the Photosynthesis P.1 complex biochemical mechanism by which plants capture sunlight February Excursion Report P.1 energy, and use it to create sugars from carbon dioxide and water. News from the ATH P.2 However, the different modes of photosynthesis are less familiar – Botanic Explorers Display P.4 they are known as C3, C4 and CAM. Upcoming Events P.6 C3 photosynthesis is by far the most common type where temperatures are moderate and water supplies are adequate. In the first step of this process, carbon dioxide is used to create a sugar molecule with three carbon atoms, hence “C3”. In warmer, drier environments, C4 photosynthesis is a more efficient Our February 2014 outing commenced difficult. Trees noted were several Ficus way of capturing sunlight energy. very pleasantly around the barbeque area and Cassia species both native and In these plants ( e.g. maize), the east of The Lagoon, with Chef Stuart exotic, including a beautifully captured carbon dioxide is used to turning out sausage and onion treats flowering Rainbow Cassia – hybrid of create an organic acid molecule better than Bunnings ever offered. We C. fistula and C. javanica , Tuckeroo with four carbon atoms (C4), discussed future outings and activities which is transported to distinctive (Cupaniopsis anacardioides) , a Syzygium during lunch and Boyd outlined the cells surrounding the leaf veins hemilamprum which Rob pointed out where the photosynthesis detailed work he has done so far on the was recovering from an attack of reactions are completed. -

Dodonaea Viscosa (Varnish-Leaf, Hopbush) Syn: D
Dodonaea viscosa (Varnish-Leaf, Hopbush) Syn: D. angustifolia The Varnish-Leaf is a fast growing evergreen shrub that can grow 2-3 m tall . The shiny leaves give a nice a decorative appearance. The elliptic leaf has a coating which protects it from loosing water. The flower is yellow without true petals and appearing in a cluster. This shrub blooms in the spring and fall, and flowers on the same plant may be male, female, or perfect. The three parted seed pods have 3 or 4 rounded wings. The plant used as a specimen hedge or background plant screen. It is can also be grown in fence rows and is interesting when espaliered. It can tolerate dry sandy or rocky condition, salt spray, wind and drought condition. Landscape Information Pronounciation: doe-DOE-nee-uh viss-KOE- suh Plant Type: Shrub Origin: Florida Heat Zones: 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16 Hardiness Zones: 9, 10, 11, 12, 13 Uses: Screen, Espalier, Specimen, Wildlife Size/Shape Growth Rate: Moderate Tree Shape: oval Canopy Symmetry: Symmetrical Canopy Density: Medium Plant Image Canopy Texture: Fine Height at Maturity: 1.5 to 3 m Spread at Maturity: 1.5 to 3 meters Time to Ultimate Height: 2 to 5 Years Dodonaea viscosa (Varnish-Leaf, Hopbush) Botanical Description Foliage Leaf Arrangement: Alternate Leaf Venation: Pinnate Leaf Persistance: Evergreen Leaf Type: Simple Leaf Blade: Less than 5 Leaf Shape: Spatulate Leaf Margins: Entire Leaf Textures: Rough Flower Image Leaf Scent: Unpleasant Color(growing season): Green Color(changing season): Green Flower Flower Showiness: