On Gran Canaria, Canary Islands, Spain
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Reptile Collection Application
Utah Division of Wildlife Resources Application for Reptile Collection Certificate of Registration Applications must be received through mail, email, or in person at the DWR Salt Lake Office Only by January 19, 2018 5:00 pm. Utah Division of Wildlife Resources, ATTN: Wildlife Registration Office, PO Box 146301; 1594 W North Temple, Ste. 2110; Salt Lake City, UT 84114-6301 or email [email protected]. There is a $10.00 non-refundable handling fee and must be included when submitting application. You may enclose a check or money order payable to DWR, pay in person at a Division Office or arrange to make a credit card payment over the phone. Please list your phone number here to arrange payment by phone. The $75.00 Certificate of Registration fee will be collected from successful applicants upon approval. If necessary, a drawing will be conducted for those species that have more applications than available permits on January 24. Remaining permits will be available January 25 on a first-come, first-served basis at the DWR Salt Lake Office. Name________________________________ Phone_______________________ Email________________________ Address____________________________ City___________________ State________ ZIP________________ Date of Birth___________________________ Limit Total Collection Species per Authorized County Permits Season County California Kingsnake 6 2 Garfield, Kane, and San Juan March 1 - Oct 31 Daggett, Duchesne, Salt Lake, Summit, Smooth Greensnake 6 1 March 1 - Oct 31 Utah, Uintah, Wasatch, and Weber Beaver, Daggett, -

A Review of Southern Iraq Herpetofauna
Vol. 3 (1): 61-71, 2019 A Review of Southern Iraq Herpetofauna Nadir A. Salman Mazaya University College, Dhi Qar, Iraq *Corresponding author: [email protected] Abstract: The present review discussed the species diversity of herpetofauna in southern Iraq due to their scientific and national interests. The review includes a historical record for the herpetofaunal studies in Iraq since the earlier investigations of the 1920s and 1950s along with the more recent taxonomic trials in the following years. It appeared that, little is known about Iraqi herpetofauna, and no comprehensive checklist has been done for these species. So far, 96 species of reptiles and amphibians have been recorded from Iraq, but only a relatively small proportion of them occur in the southern marshes. The marshes act as key habitat for globally endangered species and as a potential for as yet unexplored amphibian and reptile diversity. Despite the lack of precise localities, the tree frog Hyla savignyi, the marsh frog Pelophylax ridibunda and the green toad Bufo viridis are found in the marshes. Common reptiles in the marshes include the Caspian terrapin (Clemmys caspia), the soft-shell turtle (Trionyx euphraticus), the Euphrates softshell turtle (Rafetus euphraticus), geckos of the genus Hemidactylus, two species of skinks (Trachylepis aurata and Mabuya vittata) and a variety of snakes of the genus Coluber, the spotted sand boa (Eryx jaculus), tessellated water snake (Natrix tessellata) and Gray's desert racer (Coluber ventromaculatus). More recently, a new record for the keeled gecko, Cyrtopodion scabrum and the saw-scaled viper (Echis carinatus sochureki) was reported. The IUCN Red List includes six terrestrial and six aquatic amphibian species. -

Survival Method and Habitat of Common Wolf Snake Dr
International Journal of Engineering, Science and Mathematics Vol. 6 Issue 3, July 2017, ISSN: 2320-0294 Impact Factor: 6.765 Journal Homepage: http://www.ijesm.co.in, Email: [email protected] Double-Blind Peer Reviewed Refereed Open Access International Journal - Included in the International Serial Directories Indexed & Listed at: Ulrich's Periodicals Directory ©, U.S.A., Open J-Gage as well as in Cabell’s Directories of Publishing Opportunities, U.S.A Survival method and habitat of Common Wolf snake Dr. Pushpa Kumari PhD. (Zoology) Calorx Teachers' University, Ahmedabad Abstract A new species of the genus Lycodon Fitzinger, 1826 is described from the Cardamom Mountains of southwest Cambodia.Lycodon zoosvictoriae distinctly differs from all other species of Lycodon in Southeast Asia by a combination of its morphometric characters and unique coloration. The new species has 17 dorsal scales at midbody; 2+2 temporals; 8 supralabials; 10 infralabials; loreal separated from internasal and orbit; 213 ventrals; 85 subcaudals; pale tan brown ground color,irregular dark brown blotches on anterior part, 31 transverse blotches on posterior part of body and 26 blotches on tail.Given its submontane type locality, the new species could prove to be endemic to the Cardamom Mountains of southwestCambodia and probably Southeast Thailand. Key words: herpetofauna, Pursat, Indochina, Lycodon Introduction A new species of wolf snake is described from the tropical mixed dry deciduous forests of the Anaikatti Hills, Western Ghats, India. This species is distinct from its congeners of the Indian mainland by the following combination of characters: dorsal scales in 17:17:15 rows, scales smooth with single apical pit; anterior nasal shield larger than the posterior, loreal in contact with the internasal, but not with the eye; higher number of ventrals (210 - 224) which do not angulate laterally and hemipenis not forked at the tip and lacks spines. -

Snake Charming and the Exploitation of Snakes in Morocco
Snake charming and the exploitation of snakes in Morocco J UAN M. PLEGUEZUELOS,MÓNICA F ERICHE,JOSÉ C. BRITO and S OUMÍA F AHD Abstract Traditional activities that potentially threaten bio- also for clothing, tools, medicine and pets, as well as in diversity represent a challenge to conservationists as they try magic and religious activities (review in Alves & Rosa, to reconcile the cultural dimensions of such activities. ). Vertebrates, particularly reptiles, have frequently Quantifying the impact of traditional activities on biodiver- been used for traditional medicine. Alves et al. () iden- sity is always helpful for decision making in conservation. In tified reptile species ( families, genera) currently the case of snake charming in Morocco, the practice was in- used in traditional folk medicine, % of which are included troduced there years ago by the religious order the on the IUCN Red List (IUCN, ) and/or the CITES Aissawas, and is now an attraction in the country’s growing Appendices (CITES, ). Among the reptile species tourism industry. As a consequence wild snake populations being used for medicine, % are snakes. may be threatened by overexploitation. The focal species for Snakes have always both fascinated and repelled people, snake charming, the Egyptian cobra Naja haje, is undergo- and the reported use of snakes in magic and religious activ- ing both range and population declines. We estimated the ities is global (Alves et al., ). The sacred role of snakes level of exploitation of snakes based on field surveys and may be related to a traditional association with health and questionnaires administered to Aissawas during – eternity in some cultures (Angeletti et al., ) and many , and compared our results with those of a study con- species are under pressure from exploitation as a result ducted years previously. -

Khalladi-Bpp Anexes-Arabic.Pdf
Khalladi Windfarm and Power Line Projects Biodiversity Protection Plan, July 2015 107 Khalladi Windfarm and Power Line Projects Biodiversity Protection Plan, July 2015 108 Khalladi Windfarm and Power Line Projects Biodiversity Protection Plan, July 2015 109 Khalladi Windfarm and Power Line Projects Biodiversity Protection Plan, July 2015 110 Khalladi Windfarm and Power Line Projects Biodiversity Protection Plan, July 2015 111 Khalladi Windfarm and Power Line Projects Biodiversity Protection Plan, July 2015 112 Khalladi Windfarm and Power Line Projects Biodiversity Protection Plan, July 2015 113 The IUCN Red List Categories and Criteria are intended to be an easily and widely understood system for classifying species at high risk of global extinction. The IUCN Red List is categorized in the following Categories: • Extinct (EX): A taxon is Extinct when there is no reasonable doubt that the last individual has died. A taxon is presumed Extinct when exhaustive surveys in known and/or expected habitat, at appropriate times (diurnal, seasonal, annual), throughout its historic range have failed to record an individual. Surveys should be over a time frame appropriate to the taxon’s life cycle and life form. Khalladi Windfarm and Power Line Projects 114 Biodiversity Protection Plan, July 2015 • Extinct in the Wild (EW): A taxon is Extinct in the Wild when it is known only to survive in cultivation, in captivity or as a naturalized population (or populations) well outside the past range. A taxon is presumed Extinct in the Wild when exhaustive surveys in known and/or expected habitat, at appropriate times (diurnal, seasonal, annual), throughout its historic range have failed to record an individual. -
B I R D W a T C H I N G •
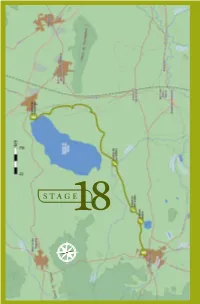
18 . F UENTE DE PIEDRA - CAMPILLOS STAGE18 E S N O 158 BIRDWATCHING • GR-249 Great Path of Malaga F UENTE DE PIEDRA - CAMPILLOS 18 . STAGE 18 Fuente de Piedra - Campillos L O C A T I O N he José Antonio Valverde Visitor´s Centre at the TReserva Natural de la Laguna de Fuente de Piedra is the starting point of Stage 18. Taking the direction south around the taken up mainly by olive trees and grain. eastern side of the salt water lagoon This type of environment will continue to you will be walking through farmland the end of this stage and it determines until the end of this stage in Campillos the species of birds which can be seen village. The 15, 7 km of Stage 18 will here. You will be crossing a stream and allow you to discover this wetland well then walking along the two lakes which known at national level in Spain, and will make your Stage 18 bird list fill cultivated farmland which creates a up with highly desirable species. The steppe-like environment. combination of wetland and steppe creates very valuable habitats with DESCRIPTION a rare composition of taxa unique at European level. ABOUT THE BIRDLIFE: Stage 18 begins at the northern tip HIGHLIGHTED SPECIES of the lagoon where you take direction Neither the length, diffi culty level or south through agricultural environment, elevation gain of this stage is particularly DID YOU KNOW? ecilio Garcia de la Leña, in Conversation 9th of “Historical Conversations of Malaga” published by Cristóbal Medina Conde (1726-1798) and entitled «About Animal Kingdom of Malaga and some Places in its Bishopric», Ccomments: «…In some lagoons, along sea shores and river banks some large and beautiful birds breed, called Flamingos and Phoenicopteros according to the ancients...», after a description of the bird´s anatomy he adds: «The Romans appreciated the bird greatly, especially its tongue which was served as an exquisite dish…». -

Cfreptiles & Amphibians
HTTPS://JOURNALS.KU.EDU/REPTILESANDAMPHIBIANSTABLE OF CONTENTS IRCF REPTILES & AMPHIBIANSREPTILES • VOL &15, AMPHIBIANS NO 4 • DEC 2008 • 28(1):157–158189 • APR 2021 IRCF REPTILES & AMPHIBIANS CONSERVATION AND NATURAL HISTORY TABLE OF CONTENTS FEATUREPredation ARTICLES on a Common Wolfsnake, . Chasing Bullsnakes (Pituophis catenifer sayi) in Wisconsin: LycodonOn the Road to aulicusUnderstanding the Ecology (Colubridae),and Conservation of the Midwest’s Giant Serpent ...................... by anJoshua M. KapferIndian 190 . The Shared History of Treeboas (Corallus grenadensis) and Humans on Grenada: Roller,A Hypothetical Coracias Excursion ............................................................................................................................ benghalensis (Coraciidae),Robert W. Henderson 198 RESEARCH ARTICLES in. The the Texas Horned Sathyamangalam Lizard in Central and Western Texas ....................... Emily Henry, JasonTiger Brewer, Krista Mougey, Reserve, and Gad Perry 204 . The Knight Anole (Anolis equestris) in Florida .............................................TamilBrian J. Camposano, Kenneth Nadu, L. Krysko, Kevin M. Enge,India Ellen M. Donlan, and Michael Granatosky 212 CONSERVATION ALERT . World’s Mammals in Crisis ...............................................................................................................................Sreedharan Nair Vishnu and Chinnasamy Ramesh .............................. 220 . More Than Mammals ..................................................................................................................................................................... -

Digestive Ecology of Two Omnivorous Canarian Lizard Species
Digestiveecology of two omnivorous Canarian lizardspecies (Gallotia,Lacertidae) AlfredoV alido 1,ManuelNogales Departamento deBiología Animal (Zoología), Universidadde La Laguna,E-38206, Tenerife, Canary Islands, Spain 1Authorfor reprint request; current address: Dept. of Ecologyand Genetics, AarhusUniversity, Ny Munkegade Building540 DK-8000 Aarhus C, Denmark e-mail: [email protected] Abstract. Omnivorousendemic Canarianlacertids ( Gallotiaatlantica and G. galloti)donotpresent any speci c digestiveand physiological adaptations to herbivorous diet, compared to species andpopulations with a different degree ofherbivory in the Canarian archipelago. The only characteristics thatcould be related tothe type of diet were thenumber of cusps per tooth (between species) andthe number of small stonescontained in droppings (between species andpopulations). The rest ofmeasured traits were correlated withlizard size andfor this reason G. galloti has longerintestines, heavier stomachs andlivers, more teethand cusps, and longer gut passage. These data suggestthat body size isamajor determinantof the reliance onplant food (mainly eshyfruits) in these lizards andfacilitates mutualisticinteractions with eshy-fruitedplant species. Introduction Structuraland functional adaptations of the digestive system for optimalprocessing of plantmatter have been described for severalomnivorous and herbivorous vertebrates (see reviewsin Lö nnberg, 1902; Ziswiler and Farner, 1972; Skoczylas, 1978; Chivers and Hladik,1980; Guard, 1980; Sibly, 1981; Jordano, -

Genus Lycodon)
Zoologica Scripta Multilocus phylogeny reveals unexpected diversification patterns in Asian wolf snakes (genus Lycodon) CAMERON D. SILER,CARL H. OLIVEROS,ANSSI SANTANEN &RAFE M. BROWN Submitted: 6 September 2012 Siler, C. D., Oliveros, C. H., Santanen, A., Brown, R. M. (2013). Multilocus phylogeny Accepted: 8 December 2012 reveals unexpected diversification patterns in Asian wolf snakes (genus Lycodon). —Zoologica doi:10.1111/zsc.12007 Scripta, 42, 262–277. The diverse group of Asian wolf snakes of the genus Lycodon represents one of many poorly understood radiations of advanced snakes in the superfamily Colubroidea. Outside of three species having previously been represented in higher-level phylogenetic analyses, nothing is known of the relationships among species in this unique, moderately diverse, group. The genus occurs widely from central to Southeast Asia, and contains both widespread species to forms that are endemic to small islands. One-third of the diversity is found in the Philippine archipelago. Both morphological similarity and highly variable diagnostic characters have contributed to confusion over species-level diversity. Additionally, the placement of the genus among genera in the subfamily Colubrinae remains uncertain, although previous studies have supported a close relationship with the genus Dinodon. In this study, we provide the first estimate of phylogenetic relationships within the genus Lycodon using a new multi- locus data set. We provide statistical tests of monophyly based on biogeographic, morpho- logical and taxonomic hypotheses. With few exceptions, we are able to reject many of these hypotheses, indicating a need for taxonomic revisions and a reconsideration of the group's biogeography. Mapping of color patterns on our preferred phylogenetic tree suggests that banded and blotched types have evolved on multiple occasions in the history of the genus, whereas the solid-color (and possibly speckled) morphotype color patterns evolved only once. -

Tail Breakage and Predatory Pressure Upon Two Invasive Snakes (Serpentes: Colubridae) at Two Islands in the Western Mediterranean
Canadian Journal of Zoology Tail breakage and predatory pressure upon two invasive snakes (Serpentes: Colubridae) at two islands in the Western Mediterranean Journal: Canadian Journal of Zoology Manuscript ID cjz-2020-0261.R2 Manuscript Type: Article Date Submitted by the 17-Jan-2021 Author: Complete List of Authors: Febrer-Serra, Maria; University of the Balearic Islands Lassnig, Nil; University of the Balearic Islands Colomar, Victor; Consorci per a la Recuperació de la Fauna de les Illes Balears Draft Sureda Gomila, Antoni; University of the Balearic Islands; Carlos III Health Institute, CIBEROBC Pinya Fernández, Samuel; University of the Balearic Islands, Biology Is your manuscript invited for consideration in a Special Not applicable (regular submission) Issue?: Zamenis scalaris, Hemorrhois hippocrepis, invasive snakes, predatory Keyword: pressure, Balearic Islands, frequency of tail breakage © The Author(s) or their Institution(s) Page 1 of 34 Canadian Journal of Zoology 1 Tail breakage and predatory pressure upon two invasive snakes (Serpentes: 2 Colubridae) at two islands in the Western Mediterranean 3 4 M. Febrer-Serra, N. Lassnig, V. Colomar, A. Sureda, S. Pinya* 5 6 M. Febrer-Serra. Interdisciplinary Ecology Group. University of the Balearic Islands, 7 Ctra. Valldemossa km 7.5, 07122 Palma, Balearic Islands, Spain. E-mail address: 8 [email protected]. 9 N. Lassnig. Interdisciplinary Ecology Group. University of the Balearic Islands, Ctra. 10 Valldemossa km 7.5, 07122 Palma, Balearic Islands, Spain. E-mail address: 11 [email protected]. 12 V. Colomar. Consortium for the RecoveryDraft of Fauna of the Balearic Islands (COFIB). 13 Government of the Balearic Islands, Spain. -

Observations of Marine Swimming in Malpolon Monspessulanus
Herpetology Notes, volume 14: 593-596 (2021) (published online on 29 March 2021) Snake overboard! Observations of marine swimming in Malpolon monspessulanus Grégory Deso1,*, Xavier Bonnet2, Cornélius De Haan3, Gilles Garnier4, Nicolas Dubos5, and Jean-Marie Ballouard6 The ability to swim is widespread in snakes and not The diversity of fundamentally terrestrial snake restricted to aquatic (freshwater) or marine species species that colonised islands worldwide, including (Jayne, 1985, 1988). A high tolerance to hypernatremia remote ones (e.g., the Galapagos Archipelago), shows (i.e., high concentration of sodium in the blood) enables that these reptiles repeatedly achieved very long and some semi-aquatic freshwater species to use brackish successful trips across the oceans (Thomas, 1997; and saline habitats. In coastal populations, snakes may Martins and Lillywhite, 2019). However, we do not even forage at sea (Tuniyev et al., 2011; Brischoux and know how snakes reached remote islands. Did they Kornilev, 2014). Terrestrial species, however, are rarely actively swim, merely float at the surface, or were observed in the open sea. While snakes are particularly they transported by drifting rafts? We also ignore the resistant to fasting (Secor and Diamond, 2000), long trips mechanisms that enable terrestrial snakes to survive in the marine environment are metabolically demanding during long periods at sea and how long they can do so. (prolonged locomotor efforts entail substantial energy Terrestrial tortoises travel hundreds of kilometres, can expense) and might be risky (Lillywhite, 2014). float for weeks in ocean currents, and can sometimes Limited freshwater availability might compromise the survive in hostile conditions without food and with hydromineral balance of individuals, even in amphibious limited access to freshwater (Gerlach et al., 2006). -

Gallotia Galloti Palmae, Fam
CITE THIS ARTCILE AS “IN PRESS” Basic and Applied Herpetology 00 (0000) 000-000 Chemical discrimination of pesticide-treated grapes by lizards (Gallotia galloti palmae, Fam. Lacertidae) Nieves Rosa Yanes-Marichal1, Angel Fermín Francisco-Sánchez1, Miguel Molina-Borja2* 1 Laboratorio de Agrobiología, Cabildo Insular de La Palma. 2 Grupo Etología y Ecología del Comportamiento, Departamento de Biología Animal, Facultad de Biología, Universidad de La Laguna, 38206 La Laguna, Tenerife, Canary Islands. * Correspondence: Phone: +34 922318341, Fax: +34 922318311, Email: [email protected] Received: 14 November 2016; returned for review: 1 December 2016; accepted 3 January 2017 Lizards from the Canary Islands may act as pests of several cultivated plants. As a case in point, vineyard farmers often complain about the lizards’ impact on grapes. Though no specific pesticide is used for lizards, several pesticides are used in vineyards to control for insects, fungi, etc. We therefore tested whether lizards (Gallotia galloti palmae) could detect and discriminate pesticide- treated from untreated grapes. To answer this question, we performed experiments with adults of both sexes obtained from three localities in La Palma Island. Two of them were a vineyard and a banana plantation that had been treated with pesticides and the other one was in a natural (untreated) site. In the laboratory, lizards were offered simultaneously one untreated (water sprayed) and one treated (with Folithion 50 LE, diluted to 0.1%) grape placed on small plates. The behaviour of the lizards towards the fruits was filmed and subsequently quantified by means of their tongue-flick, licks or bite rates to each of the grapes.