Invasive and Non-Native Aquatic Invertebrates in the Lower Coos
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Zootaxa, Mollusca, Goniodorididae, Okenia
ZOOTAXA 695 Further species of the opisthobranch genus Okenia (Nudibranchia: Goniodorididae) from the Indo-West Pacific W.B. RUDMAN Magnolia Press Auckland, New Zealand W.B. RUDMAN Further species of the opisthobranch genus Okenia (Nudibranchia: Goniodorididae) from the Indo-West Pacific (Zootaxa 695) 70 pp.; 30 cm. 25 October 2004 ISBN 1-877354-68-6 (Paperback) ISBN 1-877354-69-4 (Online edition) FIRST PUBLISHED IN 2004 BY Magnolia Press P.O. Box 41383 Auckland 1030 New Zealand e-mail: [email protected] http://www.mapress.com/zootaxa/ © 2004 Magnolia Press All rights reserved. No part of this publication may be reproduced, stored, transmitted or disseminated, in any form, or by any means, without prior written permission from the publisher, to whom all requests to reproduce copyright material should be directed in writing. This authorization does not extend to any other kind of copying, by any means, in any form, and for any purpose other than private research use. ISSN 1175-5326 (Print edition) ISSN 1175-5334 (Online edition) Zootaxa 695: 1–70 (2004) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ ZOOTAXA 695 Copyright © 2004 Magnolia Press ISSN 1175-5334 (online edition) Further species of the opisthobranch genus Okenia (Nudibranchia: Goniodorididae) from the Indo-West Pacific W.B. RUDMAN The Australian Museum, 6 College St., Sydney, NSW 2010, Australia. E-mail: [email protected]. Table of Contents Abstract . 3 Introduction. 4 Materials and Methods . 5 Descriptions . 5 Okenia Menke, 1830. 5 Okenia echinata Baba, 1949. 6 Okenia purpurata sp. nov. 9 Okenia vena sp. nov.. 13 Okenia virginiae Gosliner, 2004. -
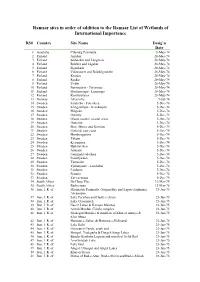
Ramsar Sites in Order of Addition to the Ramsar List of Wetlands of International Importance
Ramsar sites in order of addition to the Ramsar List of Wetlands of International Importance RS# Country Site Name Desig’n Date 1 Australia Cobourg Peninsula 8-May-74 2 Finland Aspskär 28-May-74 3 Finland Söderskär and Långören 28-May-74 4 Finland Björkör and Lågskär 28-May-74 5 Finland Signilskär 28-May-74 6 Finland Valassaaret and Björkögrunden 28-May-74 7 Finland Krunnit 28-May-74 8 Finland Ruskis 28-May-74 9 Finland Viikki 28-May-74 10 Finland Suomujärvi - Patvinsuo 28-May-74 11 Finland Martimoaapa - Lumiaapa 28-May-74 12 Finland Koitilaiskaira 28-May-74 13 Norway Åkersvika 9-Jul-74 14 Sweden Falsterbo - Foteviken 5-Dec-74 15 Sweden Klingavälsån - Krankesjön 5-Dec-74 16 Sweden Helgeån 5-Dec-74 17 Sweden Ottenby 5-Dec-74 18 Sweden Öland, eastern coastal areas 5-Dec-74 19 Sweden Getterön 5-Dec-74 20 Sweden Store Mosse and Kävsjön 5-Dec-74 21 Sweden Gotland, east coast 5-Dec-74 22 Sweden Hornborgasjön 5-Dec-74 23 Sweden Tåkern 5-Dec-74 24 Sweden Kvismaren 5-Dec-74 25 Sweden Hjälstaviken 5-Dec-74 26 Sweden Ånnsjön 5-Dec-74 27 Sweden Gammelstadsviken 5-Dec-74 28 Sweden Persöfjärden 5-Dec-74 29 Sweden Tärnasjön 5-Dec-74 30 Sweden Tjålmejaure - Laisdalen 5-Dec-74 31 Sweden Laidaure 5-Dec-74 32 Sweden Sjaunja 5-Dec-74 33 Sweden Tavvavuoma 5-Dec-74 34 South Africa De Hoop Vlei 12-Mar-75 35 South Africa Barberspan 12-Mar-75 36 Iran, I. R. -

Striped Bass Morone Saxatilis
COSEWIC Assessment and Status Report on the Striped Bass Morone saxatilis in Canada Southern Gulf of St. Lawrence Population St. Lawrence Estuary Population Bay of Fundy Population SOUTHERN GULF OF ST. LAWRENCE POPULATION - THREATENED ST. LAWRENCE ESTUARY POPULATION - EXTIRPATED BAY OF FUNDY POPULATION - THREATENED 2004 COSEWIC COSEPAC COMMITTEE ON THE STATUS OF COMITÉ SUR LA SITUATION ENDANGERED WILDLIFE DES ESPÈCES EN PÉRIL IN CANADA AU CANADA COSEWIC status reports are working documents used in assigning the status of wildlife species suspected of being at risk. This report may be cited as follows: COSEWIC 2004. COSEWIC assessment and status report on the Striped Bass Morone saxatilis in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. vii + 43 pp. (www.sararegistry.gc.ca/status/status_e.cfm) Production note: COSEWIC would like to acknowledge Jean Robitaille for writing the status report on the Striped Bass Morone saxatilis prepared under contract with Environment Canada, overseen and edited by Claude Renaud the COSEWIC Freshwater Fish Species Specialist Subcommittee Co-chair. For additional copies contact: COSEWIC Secretariat c/o Canadian Wildlife Service Environment Canada Ottawa, ON K1A 0H3 Tel.: (819) 997-4991 / (819) 953-3215 Fax: (819) 994-3684 E-mail: COSEWIC/[email protected] http://www.cosewic.gc.ca Ếgalement disponible en français sous le titre Ếvaluation et Rapport de situation du COSEPAC sur la situation de bar rayé (Morone saxatilis) au Canada. Cover illustration: Striped Bass — Drawing from Scott and Crossman, 1973. Her Majesty the Queen in Right of Canada 2004 Catalogue No. CW69-14/421-2005E-PDF ISBN 0-662-39840-8 HTML: CW69-14/421-2005E-HTML 0-662-39841-6 Recycled paper COSEWIC Assessment Summary Assessment Summary – November 2004 Common name Striped Bass (Southern Gulf of St. -

A Radical Solution: the Phylogeny of the Nudibranch Family Fionidae
RESEARCH ARTICLE A Radical Solution: The Phylogeny of the Nudibranch Family Fionidae Kristen Cella1, Leila Carmona2*, Irina Ekimova3,4, Anton Chichvarkhin3,5, Dimitry Schepetov6, Terrence M. Gosliner1 1 Department of Invertebrate Zoology, California Academy of Sciences, San Francisco, California, United States of America, 2 Department of Marine Sciences, University of Gothenburg, Gothenburg, Sweden, 3 Far Eastern Federal University, Vladivostok, Russia, 4 Biological Faculty, Moscow State University, Moscow, Russia, 5 A.V. Zhirmunsky Instutute of Marine Biology, Russian Academy of Sciences, Vladivostok, Russia, 6 National Research University Higher School of Economics, Moscow, Russia a11111 * [email protected] Abstract Tergipedidae represents a diverse and successful group of aeolid nudibranchs, with approx- imately 200 species distributed throughout most marine ecosystems and spanning all bio- OPEN ACCESS geographical regions of the oceans. However, the systematics of this family remains poorly Citation: Cella K, Carmona L, Ekimova I, understood since no modern phylogenetic study has been undertaken to support any of the Chichvarkhin A, Schepetov D, Gosliner TM (2016) A Radical Solution: The Phylogeny of the proposed classifications. The present study is the first molecular phylogeny of Tergipedidae Nudibranch Family Fionidae. PLoS ONE 11(12): based on partial sequences of two mitochondrial (COI and 16S) genes and one nuclear e0167800. doi:10.1371/journal.pone.0167800 gene (H3). Maximum likelihood, maximum parsimony and Bayesian analysis were con- Editor: Geerat J. Vermeij, University of California, ducted in order to elucidate the systematics of this family. Our results do not recover the tra- UNITED STATES ditional Tergipedidae as monophyletic, since it belongs to a larger clade that includes the Received: July 7, 2016 families Eubranchidae, Fionidae and Calmidae. -

Bryozoan Genera Fenestrulina and Microporella No Longer Confamilial; Multi-Gene Phylogeny Supports Separation
Zoological Journal of the Linnean Society, 2019, 186, 190–199. With 2 figures. Bryozoan genera Fenestrulina and Microporella no longer confamilial; multi-gene phylogeny supports separation RUSSELL J. S. ORR1*, ANDREA WAESCHENBACH2, EMILY L. G. ENEVOLDSEN3, Downloaded from https://academic.oup.com/zoolinnean/article/186/1/190/5096936 by guest on 29 September 2021 JEROEN P. BOEVE3, MARIANNE N. HAUGEN3, KJETIL L. VOJE3, JOANNE PORTER4, KAMIL ZÁGORŠEK5, ABIGAIL M. SMITH6, DENNIS P. GORDON7 and LEE HSIANG LIOW1,3 1Natural History Museum, University of Oslo, Oslo, Norway 2Department of Life Sciences, Natural History Museum, London, UK 3Centre for Ecological & Evolutionary Synthesis, Department of Biosciences, University of Oslo, Oslo, Norway 4Centre for Marine Biodiversity and Biotechnology, School of Life Sciences, Heriot Watt University, Edinburgh, UK 5Department of Geography, Technical University of Liberec, Czech Republic 6Department of Marine Science, University of Otago, Dunedin, New Zealand 7National Institute of Water and Atmospheric Research, Wellington, New Zealand Received 25 March 2018; revised 28 June 2018; accepted for publication 11 July 2018 Bryozoans are a moderately diverse, mostly marine phylum with a fossil record extending to the Early Ordovician. Compared to other phyla, little is known about their phylogenetic relationships at both lower and higher taxonomic levels. Hence, an effort is being made to elucidate their phylogenetic relationships. Here, we present newly sequenced nuclear and mitochondrial genes for 21 cheilostome bryozoans. Combining these data with existing orthologous molecular data, we focus on reconstructing the phylogenetic relationships of Fenestrulina and Microporella, two species-rich genera. They are currently placed in Microporellidae, defined by having a semicircular primary orifice and a proximal ascopore. -

From Ghost and Mud Shrimp
Zootaxa 4365 (3): 251–301 ISSN 1175-5326 (print edition) http://www.mapress.com/j/zt/ Article ZOOTAXA Copyright © 2017 Magnolia Press ISSN 1175-5334 (online edition) https://doi.org/10.11646/zootaxa.4365.3.1 http://zoobank.org/urn:lsid:zoobank.org:pub:C5AC71E8-2F60-448E-B50D-22B61AC11E6A Parasites (Isopoda: Epicaridea and Nematoda) from ghost and mud shrimp (Decapoda: Axiidea and Gebiidea) with descriptions of a new genus and a new species of bopyrid isopod and clarification of Pseudione Kossmann, 1881 CHRISTOPHER B. BOYKO1,4, JASON D. WILLIAMS2 & JEFFREY D. SHIELDS3 1Division of Invertebrate Zoology, American Museum of Natural History, Central Park West @ 79th St., New York, New York 10024, U.S.A. E-mail: [email protected] 2Department of Biology, Hofstra University, Hempstead, New York 11549, U.S.A. E-mail: [email protected] 3Department of Aquatic Health Sciences, Virginia Institute of Marine Science, College of William & Mary, P.O. Box 1346, Gloucester Point, Virginia 23062, U.S.A. E-mail: [email protected] 4Corresponding author Table of contents Abstract . 252 Introduction . 252 Methods and materials . 253 Taxonomy . 253 Isopoda Latreille, 1817 . 253 Bopyroidea Rafinesque, 1815 . 253 Ionidae H. Milne Edwards, 1840. 253 Ione Latreille, 1818 . 253 Ione cornuta Bate, 1864 . 254 Ione thompsoni Richardson, 1904. 255 Ione thoracica (Montagu, 1808) . 256 Bopyridae Rafinesque, 1815 . 260 Pseudioninae Codreanu, 1967 . 260 Acrobelione Bourdon, 1981. 260 Acrobelione halimedae n. sp. 260 Key to females of species of Acrobelione Bourdon, 1981 . 262 Gyge Cornalia & Panceri, 1861. 262 Gyge branchialis Cornalia & Panceri, 1861 . 262 Gyge ovalis (Shiino, 1939) . 264 Ionella Bonnier, 1900 . -

ISOLATION and IDENTIFICATION of SECONDARY METABOLITES from the BRYOZOAN Cryptosula Zavjalovensis from HOKKAIDO, JAPAN
LOVEILLE JUN AMARILLE GONZAGA ISOLATION AND IDENTIFICATION OF SECONDARY METABOLITES FROM THE BRYOZOAN Cryptosula zavjalovensis FROM HOKKAIDO, JAPAN UNIVERSIDADE DO ALGARVE FACULDADE DE CIÊNCIAS E TECNOLOGIA 2017 LOVEILLE JUN AMARILLE GONZAGA ISOLATION AND IDENTIFICATION OF SECONDARY METABOLITES FROM THE BRYOZOAN Cryptosula zavjalovensis FROM HOKKAIDO, JAPAN Erasmus Mundus MSc in Chemical Innovation and Regulation Mestrado Erasmus Mundus em Inovação Química e Regulamentação Trabalho efetuado sob a orientação de: Work supervised by: Prof. Isabel Cavaco (Universidade do Algarve) Prof. Helena Fortunato (Hokkaido University) UNIVERSIDADE DO ALGARVE FACULDADE DE CIÊNCIAS E TECNOLOGIA 2017 DECLARATION OF AUTHORSHIP ISOLATION AND IDENTIFICATION OF SECONDARY METABOLITES FROM THE BRYOZOAN Cryptosula zavjalovensis FROM HOKKAIDO, JAPAN I declare that I am the author of this work, which is original. The work cites other authors and works, which are adequately referred in the text and are listed in the bibliography. ____________________________________ Loveille Jun A. Gonzaga Copyright: Loveille Jun A. Gonzaga. The University of Algarve has the right to keep and publicize this work through printed copies in paper of digital form, or any other means of reproduction, to disseminate it in scientific repositories and to allow its copy and distribution with educational and/or research objectives, as long as they are non- commercial and give credit to the author and editor. I ACKNOWLEDGEMENTS I would like to extend my heartfelt gratitude to everyone who have been part of my Erasmus Mundus journey. The academic part of it might be concluded with this research work but the journey continues. This work wouldn’t have been possible without the immense help of such amazing people, so I would like to take this opportunity to thank: The European Commission and the EMMC-ChIR program for giving me this opportunity to pursue a relevant and timely international master’s program. -

Upogebia Pugettensis Class: Malacostraca Order: Decapoda Section: Anomura, Paguroidea the Blue Mud Shrimp Family: Upogebiidae
Phylum: Arthropoda, Crustacea Upogebia pugettensis Class: Malacostraca Order: Decapoda Section: Anomura, Paguroidea The blue mud shrimp Family: Upogebiidae Taxonomy: Dana described Gebia (on either side of the mouth), two pairs of pugettensis in 1852 and this species was later maxillae and three pairs of maxillipeds. The redescribed as Upogebia pugettensis maxillae and maxillipeds attach posterior to (Stevens 1928; Williams 1986). the mouth and extend to cover the mandibles (Ruppert et al. 2004). Description Carapace: Bears two rows of 11–12 Size: The type specimen was 50.8 mm in teeth laterally (Fig. 1) in addition to a small length and the illustrated specimen (ovigerous distal spines (13 distal spines, 20 lateral teeth female from Coos Bay, Fig. 1) was 90 mm in on carapace shoulder, see Wicksten 2011). length. Individuals are often larger and reach Carapace with thalassinidean line extending sizes to 100 mm (range 75–112 mm) and from anterior to posterior margin (Wicksten northern specimens are larger than those in 2011). southern California (MacGinitie and Rostrum: Large, tridentate, obtuse, MacGinitie 1949; Wicksten 2011). rough and hairy (Schmitt 1921), the sides Color: Light blue green to deep olive brown bear 3–5 short conical teeth (Wicksten 2011). with brown fringes on pleopods and pleon. Rostral tip shorter than antennular peduncle. Individual color variable and may depend on Two short processes extending on either side feeding habits (see Fig. 321, Kozloff 1993; each with 0–2 dorsal teeth (Wicksten 2011). Wicksten 2011). Teeth: General Morphology: The body of decapod Pereopods: Two to five simple crustaceans can be divided into the walking legs. -

Looking Beyond the Mortality of Bycatch: Sublethal Effects of Incidental Capture on Marine Animals
Biological Conservation 171 (2014) 61–72 Contents lists available at ScienceDirect Biological Conservation journal homepage: www.elsevier.com/locate/biocon Review Looking beyond the mortality of bycatch: sublethal effects of incidental capture on marine animals a, a a,b b a Samantha M. Wilson ⇑, Graham D. Raby , Nicholas J. Burnett , Scott G. Hinch , Steven J. Cooke a Fish Ecology and Conservation Physiology Laboratory, Department of Biology and Institute of Environmental Sciences, Carleton University, Ottawa, ON, Canada b Pacific Salmon Ecology and Conservation Laboratory, Department of Forest and Conservation Sciences, University of British Columbia, Vancouver, BC, Canada article info abstract Article history: There is a widely recognized need to understand and reduce the incidental effects of marine fishing on Received 14 August 2013 non-target animals. Previous research on marine bycatch has largely focused on simply quantifying mor- Received in revised form 10 January 2014 tality. However, much less is known about the organism-level sublethal effects, including the potential Accepted 13 January 2014 for behavioural alterations, physiological and energetic costs, and associated reductions in feeding, growth, or reproduction (i.e., fitness) which can occur undetected following escape or release from fishing gear. We reviewed the literature and found 133 marine bycatch papers that included sublethal endpoints Keywords: such as physiological disturbance, behavioural impairment, injury, reflex impairment, and effects on RAMP reproduction, -

Olympia Oyster (Ostrea Lurida)
COSEWIC Assessment and Status Report on the Olympia Oyster Ostrea lurida in Canada SPECIAL CONCERN 2011 COSEWIC status reports are working documents used in assigning the status of wildlife species suspected of being at risk. This report may be cited as follows: COSEWIC. 2011. COSEWIC assessment and status report on the Olympia Oyster Ostrea lurida in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. xi + 56 pp. (www.sararegistry.gc.ca/status/status_e.cfm). Previous report(s): COSEWIC. 2000. COSEWIC assessment and status report on the Olympia Oyster Ostrea conchaphila in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. vii + 30 pp. (www.sararegistry.gc.ca/status/status_e.cfm) Gillespie, G.E. 2000. COSEWIC status report on the Olympia Oyster Ostrea conchaphila in Canada in COSEWIC assessment and update status report on the Olympia Oyster Ostrea conchaphila in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. 1-30 pp. Production note: COSEWIC acknowledges Graham E. Gillespie for writing the provisional status report on the Olympia Oyster, Ostrea lurida, prepared under contract with Environment Canada and Fisheries and Oceans Canada. The contractor’s involvement with the writing of the status report ended with the acceptance of the provisional report. Any modifications to the status report during the subsequent preparation of the 6-month interim and 2-month interim status reports were overseen by Robert Forsyth and Dr. Gerald Mackie, COSEWIC Molluscs Specialist Subcommittee Co-Chair. For additional copies contact: COSEWIC Secretariat c/o Canadian Wildlife Service Environment Canada Ottawa, ON K1A 0H3 Tel.: 819-953-3215 Fax: 819-994-3684 E-mail: COSEWIC/[email protected] http://www.cosewic.gc.ca Également disponible en français sous le titre Ếvaluation et Rapport de situation du COSEPAC sur l’huître plate du Pacifique (Ostrea lurida) au Canada. -

OREGON ESTUARINE INVERTEBRATES an Illustrated Guide to the Common and Important Invertebrate Animals
OREGON ESTUARINE INVERTEBRATES An Illustrated Guide to the Common and Important Invertebrate Animals By Paul Rudy, Jr. Lynn Hay Rudy Oregon Institute of Marine Biology University of Oregon Charleston, Oregon 97420 Contract No. 79-111 Project Officer Jay F. Watson U.S. Fish and Wildlife Service 500 N.E. Multnomah Street Portland, Oregon 97232 Performed for National Coastal Ecosystems Team Office of Biological Services Fish and Wildlife Service U.S. Department of Interior Washington, D.C. 20240 Table of Contents Introduction CNIDARIA Hydrozoa Aequorea aequorea ................................................................ 6 Obelia longissima .................................................................. 8 Polyorchis penicillatus 10 Tubularia crocea ................................................................. 12 Anthozoa Anthopleura artemisia ................................. 14 Anthopleura elegantissima .................................................. 16 Haliplanella luciae .................................................................. 18 Nematostella vectensis ......................................................... 20 Metridium senile .................................................................... 22 NEMERTEA Amphiporus imparispinosus ................................................ 24 Carinoma mutabilis ................................................................ 26 Cerebratulus californiensis .................................................. 28 Lineus ruber ......................................................................... -

Version of the Manuscript
Accepted Manuscript Antarctic and sub-Antarctic Nacella limpets reveal novel evolutionary charac- teristics of mitochondrial genomes in Patellogastropoda Juan D. Gaitán-Espitia, Claudio A. González-Wevar, Elie Poulin, Leyla Cardenas PII: S1055-7903(17)30583-3 DOI: https://doi.org/10.1016/j.ympev.2018.10.036 Reference: YMPEV 6324 To appear in: Molecular Phylogenetics and Evolution Received Date: 15 August 2017 Revised Date: 23 July 2018 Accepted Date: 30 October 2018 Please cite this article as: Gaitán-Espitia, J.D., González-Wevar, C.A., Poulin, E., Cardenas, L., Antarctic and sub- Antarctic Nacella limpets reveal novel evolutionary characteristics of mitochondrial genomes in Patellogastropoda, Molecular Phylogenetics and Evolution (2018), doi: https://doi.org/10.1016/j.ympev.2018.10.036 This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain. Version: 23-07-2018 SHORT COMMUNICATION Running head: mitogenomes Nacella limpets Antarctic and sub-Antarctic Nacella limpets reveal novel evolutionary characteristics of mitochondrial genomes in Patellogastropoda Juan D. Gaitán-Espitia1,2,3*; Claudio A. González-Wevar4,5; Elie Poulin5 & Leyla Cardenas3 1 The Swire Institute of Marine Science and School of Biological Sciences, The University of Hong Kong, Pokfulam, Hong Kong, China 2 CSIRO Oceans and Atmosphere, GPO Box 1538, Hobart 7001, TAS, Australia.