10482 2018 1112 Author
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Green-Tree Retention and Controlled Burning in Restoration and Conservation of Beetle Diversity in Boreal Forests
Dissertationes Forestales 21 Green-tree retention and controlled burning in restoration and conservation of beetle diversity in boreal forests Esko Hyvärinen Faculty of Forestry University of Joensuu Academic dissertation To be presented, with the permission of the Faculty of Forestry of the University of Joensuu, for public criticism in auditorium C2 of the University of Joensuu, Yliopistonkatu 4, Joensuu, on 9th June 2006, at 12 o’clock noon. 2 Title: Green-tree retention and controlled burning in restoration and conservation of beetle diversity in boreal forests Author: Esko Hyvärinen Dissertationes Forestales 21 Supervisors: Prof. Jari Kouki, Faculty of Forestry, University of Joensuu, Finland Docent Petri Martikainen, Faculty of Forestry, University of Joensuu, Finland Pre-examiners: Docent Jyrki Muona, Finnish Museum of Natural History, Zoological Museum, University of Helsinki, Helsinki, Finland Docent Tomas Roslin, Department of Biological and Environmental Sciences, Division of Population Biology, University of Helsinki, Helsinki, Finland Opponent: Prof. Bengt Gunnar Jonsson, Department of Natural Sciences, Mid Sweden University, Sundsvall, Sweden ISSN 1795-7389 ISBN-13: 978-951-651-130-9 (PDF) ISBN-10: 951-651-130-9 (PDF) Paper copy printed: Joensuun yliopistopaino, 2006 Publishers: The Finnish Society of Forest Science Finnish Forest Research Institute Faculty of Agriculture and Forestry of the University of Helsinki Faculty of Forestry of the University of Joensuu Editorial Office: The Finnish Society of Forest Science Unioninkatu 40A, 00170 Helsinki, Finland http://www.metla.fi/dissertationes 3 Hyvärinen, Esko 2006. Green-tree retention and controlled burning in restoration and conservation of beetle diversity in boreal forests. University of Joensuu, Faculty of Forestry. ABSTRACT The main aim of this thesis was to demonstrate the effects of green-tree retention and controlled burning on beetles (Coleoptera) in order to provide information applicable to the restoration and conservation of beetle species diversity in boreal forests. -

Envenomations in Humans Caused by The
linica f C l To o x l ic a o n r l o u g o y J Amaral et al., J Clin Toxicol 2018, 8:4 Journal of Clinical Toxicology DOI: 10.4172/2161-0495.1000392 ISSN: 2161-0495 Case Report Open Access Envenomations in Humans Caused by the Venomous Beetle Onychocerus albitarsis: Observation of Two Cases in São Paulo State, Brazil Amaral ALS1*, Castilho AL1, Borges de Sá AL2 and Haddad V Jr3 1Departamento de Zoologia, Instituto de Biociências, Universidade Estadual Paulista – UNESP, CEP 18618-000, Botucatu, São Paulo State, Brazil 2Private Clinic, Botucatu, São Paulo State, Brazil 3Departamento de Dermatologia e Radioterapia, Faculdade de Medicina, Universidade Estadual Paulista – UNESP, CP 557, CEP 18618-000, Botucatu, São Paulo State, Brazil *Corresponding author: Antonio L. Sforcin Amaral, Departamento de Zoologia, Instituto de Biociências, Universidade Estadual Paulista – UNESP, CEP 18618-000, Botucatu, São Paulo State, Brazil, Email: [email protected] Received date: July 23, 2018; Accepted date: August 21, 2018; Published date: August 24, 2018 Copyright: ©2018 Amaral ALS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Abstract Beetles (Coleoptera) are the most diverse group of animals in the world and occur in many environments. In Atlantic and Amazon rainforests, the scorpion-beetle Onychocerus albitarsis (Cerambycidae), can be found. It has venom glandules and inoculators organs in the antenna extremities. Two injuries in humans are reported, showing different patterns of skin reaction after the stings. -

11-16589 PRA Record Saperda Candida
PRA Saperda candida 11-16589 (10-15760, 09-15659) European and Mediterranean Plant Protection Organisation Organisation Européenne et Méditerranéenne pour la Protection des Plantes Guidelines on Pest Risk Analysis Decision-support scheme for quarantine pests Version N°3 Pest Risk Analysis for Saperda candida Pest risk analysts: Expert Working group for PRA for Saperda candida (met in 2009-11) ANDERSON Helen (Ms) - The Food and Environment Research Agency (GB) AGNELLO Arthur (Mr) - Department of Entomology New York State Agricultural Experiment Station(USA) BAUFELD Peter (Mr) - Julius Kühn Institut (JKI), Federal Research Centre for Cultivated Plants, Institute for National and International Plant Health (DE) GILL Bruce D. (Mr) - Head Entomology, Ottawa Plant Laboratories, C.F.I.A. (CA) PFEILSTETTER Ernst (Mr) - Julius Kühn Institut (JKI), Federal Research Centre for Cultivated Plants, Institute for National and International Plant Health (DE) (core member) STEFFEK Robert (Mr) - Austrian Agency for Health and Food Safety (AGES), Institute for Plant Health (AT) (core member) VAN DER GAAG Dirk Jan (Mr) - Plant Protection Service (NL) (core member) The Section on risk management was reviewed by the EPPO Panel on Phytosanitary Measures in 2010-02-18. 1 PRA Saperda candida Stage 1: Initiation 1 Identification of a In summer 2008, the presence of Saperda candida was detected for the first time in Germany and in Europe (Nolte & Give the reason for performing the PRA single pest Krieger, 2008). This wood boring insect was observed on the island of Fehmarn on urban trees ( Sorbus intermedia and other host plants) and eradication measures were taken against it. S. candida is considered as a pest of apple trees and other tree species in North America. -

Data on Cerambycidae and Chrysomelidae (Coleoptera: Chrysomeloidea) from Bucureªti and Surroundings
Travaux du Muséum National d’Histoire Naturelle © Novembre Vol. LI pp. 387–416 «Grigore Antipa» 2008 DATA ON CERAMBYCIDAE AND CHRYSOMELIDAE (COLEOPTERA: CHRYSOMELOIDEA) FROM BUCUREªTI AND SURROUNDINGS RODICA SERAFIM, SANDA MAICAN Abstract. The paper presents a synthesis of the data refering to the presence of cerambycids and chrysomelids species of Bucharest and its surroundings, basing on bibliographical sources and the study of the collection material. A number of 365 species of superfamily Chrysomeloidea (140 cerambycids and 225 chrysomelids species), belonging to 125 genera of 16 subfamilies are listed. The species Chlorophorus herbstii, Clytus lama, Cortodera femorata, Phytoecia caerulea, Lema cyanella, Chrysolina varians, Phaedon cochleariae, Phyllotreta undulata, Cassida prasina and Cassida vittata are reported for the first time in this area. Résumé. Ce travail présente une synthèse des données concernant la présence des espèces de cerambycides et de chrysomelides de Bucarest et de ses environs, la base en étant les sources bibliographiques ainsi que l’étude du matériel existant dans les collections du musée. La liste comprend 365 espèces appartenant à la supra-famille des Chrysomeloidea (140 espèces de cerambycides et 225 espèces de chrysomelides), encadrées en 125 genres et 16 sous-familles. Les espèces Chlorophorus herbstii, Clytus lama, Cortodera femorata, Phytoecia caerulea, Lema cyanella, Chrysolina varians, Phaedon cochleariae, Phyllotreta undulata, Cassida prasina et Cassida vittata sont mentionnées pour la première fois dans cette zone Key words: Coleoptera, Chrysomeloidea, Cerambycidae, Chrysomelidae, Bucureºti (Bucharest) and surrounding areas. INTRODUCTION Data on the distribution of the cerambycids and chrysomelids species in Bucureºti (Bucharest) and the surrounding areas were published beginning with the end of the 19th century by: Jaquet (1898 a, b, 1899 a, b, 1900 a, b, 1901, 1902), Montandon (1880, 1906, 1908), Hurmuzachi (1901, 1902, 1904), Fleck (1905 a, b), Manolache (1930), Panin (1941, 1944), Eliescu et al. -

Dothistroma Septosporum
Copyright is owned by the Author of the thesis. Permission is given for a copy to be downloaded by an individual for the purpose of research and private study only. The thesis may not be reproduced elsewhere without the permission of the Author. Secondary metabolism of the forest pathogen Dothistroma septosporum A thesis presented in the partial fulfilment of the requirements for the degree of Doctor of Philosophy (PhD) in Genetics at Massey University, Manawatu, New Zealand Ibrahim Kutay Ozturk 2016 ABSTRACT Dothistroma septosporum is a fungus causing the disease Dothistroma needle blight (DNB) on more than 80 pine species in 76 countries, and causes serious economic losses. A secondary metabolite (SM) dothistromin, produced by D. septosporum, is a virulence factor required for full disease expression but is not needed for the initial formation of disease lesions. Unlike the majority of fungal SMs whose biosynthetic enzyme genes are arranged in a gene cluster, dothistromin genes are dispersed in a fragmented arrangement. Therefore, it was of interest whether D. septosporum has other SMs that are required in the disease process, as well as having SM genes that are clustered as in other fungi. Genome sequencing of D. septosporum revealed that D. septosporum has 11 SM core genes, which is fewer than in closely related species. In this project, gene cluster analyses around the SM core genes were done to assess if there are intact or other fragmented gene clusters. In addition, one of the core SM genes, DsNps3, that was highly expressed at an early stage of plant infection, was knocked out and the phenotype of this mutant was analysed. -

Stem Damages Caused by Heart Rot and Large Poplar Borer on Hybrid and European Aspen
Forestry Studies | Metsanduslikud Uurimused, Vol. 66, Pages 21–26 Stem damages caused by heart rot and large poplar borer on hybrid and European aspen Martins Zeps, Silva Senhofa, Mara Zadina, Una Neimane and Aris Jansons* Zeps, M., Senhofa, S., Zadina, M., Neimane, U., Jansons, A. 2017. Stem damages caused by heart rot and large poplar borer on hybrid and European aspen. – Forestry Studies | Metsanduslikud Uurimused 66, 21–26. ISSN 1406-9954. Journal homepage: http://mi.emu.ee/forestry.studies Abstract. Solid wood production of hybrid aspen requires relative longer rotation peri- ods, thus increasing risk of wood damages by pests and diseases. We compared dam- ages by heart rot and poplar borer of 48 years old hybrid (Populus tremuloides Michx. × P. tremula L.) and European aspen in a progeny trial located in Eastern part of Latvia. Trees were harvested and rot patches and galleries were recorded and measured at a stump level. The number of galleries had positive relation on number of patches and total area of rot. The susceptibility of the rot and poplar borer was similar for both hybrid and European aspen. Yet, some differences among families were detected. No effect of pathogens damage was observed on the tree growth. Larger trees had smaller proportion and incidence of rot and galleries per unit of area as well as wider outer rot-free wood layer. Key words: Populus tremula, Populus tremuloides × P. tremula, wood damages, heart rot, Saperda carcharias. Authors’ address: Latvian State Forest Research Institute ‘Silava’, Rigas str. 111, Salaspils, LV-2169, Latvia; *e-mail: [email protected] Introduction increases. -

Scope: Munis Entomology & Zoology Publishes a Wide Variety of Papers
_____________ Mun. Ent. Zool. Vol. 4, No. 1, January 2009___________ I MUNIS ENTOMOLOGY & ZOOLOGY Ankara / Turkey II _____________ Mun. Ent. Zool. Vol. 4, No. 1, January 2009___________ Scope: Munis Entomology & Zoology publishes a wide variety of papers on all aspects of Entomology and Zoology from all of the world, including mainly studies on systematics, taxonomy, nomenclature, fauna, biogeography, biodiversity, ecology, morphology, behavior, conservation, paleobiology and other aspects are appropriate topics for papers submitted to Munis Entomology & Zoology. Submission of Manuscripts: Works published or under consideration elsewhere (including on the internet) will not be accepted. At first submission, one double spaced hard copy (text and tables) with figures (may not be original) must be sent to the Editors, Dr. Hüseyin Özdikmen for publication in MEZ. All manuscripts should be submitted as Word file or PDF file in an e-mail attachment. If electronic submission is not possible due to limitations of electronic space at the sending or receiving ends, unavailability of e-mail, etc., we will accept “hard” versions, in triplicate, accompanied by an electronic version stored in a floppy disk, a CD-ROM. Review Process: When submitting manuscripts, all authors provides the name, of at least three qualified experts (they also provide their address, subject fields and e-mails). Then, the editors send to experts to review the papers. The review process should normally be completed within 45-60 days. After reviewing papers by reviwers: Rejected papers are discarded. For accepted papers, authors are asked to modify their papers according to suggestions of the reviewers and editors. Final versions of manuscripts and figures are needed in a digital format. -
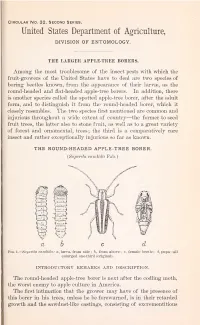
The Larger Apple-Tree Borers
Circular No. 32, Second Series. United States Department of Agriculture, DIVISION OF ENTOMOLOGY. THE LARGER APPLE-TREE BORERS. Among the most troublesome of the insect pests with which the fruit-growers of the United States have to deal are two species of boring beetles known, from the appearance of their larvse, as the round-headed and flat-headed apple-tree borers. In addition, there is another species called the spotted apple-tree borer, after the adult form, and to distinguish it from the round-headed borer, which it closely resembles. The two species first mentioned are common and injurious throughout a wide extent of country—the former to seed fruit trees, the latter also to stone fruit, as well as to a great variety of forest and ornamental, trees; the third is a comparatively rare insect and rather exceptionally injurious so far as known. THE ROUND-HEADED APPLE-TREE BORER. (Saperda Candida Fab.) Fig. 1.—Saperda Candida: a, larva, from side; b, from above; c, female beetle; d, pupa—all enlarged one-third (original). INTRODUCTORY REMARKS AND DESCRIPTION. The round-headed apple-tree borer is next after the codling moth, the worst enemy to apple culture in America. The first intimation that the grower may have of the presence of this borer in his trees, unless he be forewarned, is in their retarded growth and the sawdust-like castings, consisting of excrementitious matter and gnawings of woody fiber, which the larvae extrude from openings into their burrows. This manifestation is usually accom- panied by more or less evident discoloration of the bark, and, in early spring, particularly, slight exudation of sap. -

A Higher-Level Phylogenetic Classification of the Fungi
mycological research 111 (2007) 509–547 available at www.sciencedirect.com journal homepage: www.elsevier.com/locate/mycres A higher-level phylogenetic classification of the Fungi David S. HIBBETTa,*, Manfred BINDERa, Joseph F. BISCHOFFb, Meredith BLACKWELLc, Paul F. CANNONd, Ove E. ERIKSSONe, Sabine HUHNDORFf, Timothy JAMESg, Paul M. KIRKd, Robert LU¨ CKINGf, H. THORSTEN LUMBSCHf, Franc¸ois LUTZONIg, P. Brandon MATHENYa, David J. MCLAUGHLINh, Martha J. POWELLi, Scott REDHEAD j, Conrad L. SCHOCHk, Joseph W. SPATAFORAk, Joost A. STALPERSl, Rytas VILGALYSg, M. Catherine AIMEm, Andre´ APTROOTn, Robert BAUERo, Dominik BEGEROWp, Gerald L. BENNYq, Lisa A. CASTLEBURYm, Pedro W. CROUSl, Yu-Cheng DAIr, Walter GAMSl, David M. GEISERs, Gareth W. GRIFFITHt,Ce´cile GUEIDANg, David L. HAWKSWORTHu, Geir HESTMARKv, Kentaro HOSAKAw, Richard A. HUMBERx, Kevin D. HYDEy, Joseph E. IRONSIDEt, Urmas KO˜ LJALGz, Cletus P. KURTZMANaa, Karl-Henrik LARSSONab, Robert LICHTWARDTac, Joyce LONGCOREad, Jolanta MIA˛ DLIKOWSKAg, Andrew MILLERae, Jean-Marc MONCALVOaf, Sharon MOZLEY-STANDRIDGEag, Franz OBERWINKLERo, Erast PARMASTOah, Vale´rie REEBg, Jack D. ROGERSai, Claude ROUXaj, Leif RYVARDENak, Jose´ Paulo SAMPAIOal, Arthur SCHU¨ ßLERam, Junta SUGIYAMAan, R. Greg THORNao, Leif TIBELLap, Wendy A. UNTEREINERaq, Christopher WALKERar, Zheng WANGa, Alex WEIRas, Michael WEISSo, Merlin M. WHITEat, Katarina WINKAe, Yi-Jian YAOau, Ning ZHANGav aBiology Department, Clark University, Worcester, MA 01610, USA bNational Library of Medicine, National Center for Biotechnology Information, -

(Insects). Note 1
Muzeul Olteniei Craiova. Oltenia. Studii úi comunicări. ùtiinĠele Naturii. Tom. 29, No. 2/2013 ISSN 1454-6914 CONTRIBUTIONS TO THE KNOWLEDGE OF RESEARCH ON BEETLE PARASITE FAUNA (INSECTS). NOTE 1. LILA Gima Abstract. The paper presents a synthesis of the data on the parasite fauna of longhorn beetles taken from papers published between 1959 and 2009 ((CONSTANTINEANU, 1959; PANIN &SĂVULESCU, 1961; BALTHASAR, 1963; TUDOR, 1969; PISICĂ, 2001; PISICĂ &POPESCU, 2009) and abroad, between 1945-1966 (GYORFI, 1945-1947; BALACHOWSKY, 1962-1963; HURPIN 1962 - for parasites and parasitoids species found in species of beetles and GRASSE 1953 and KUDO 1966 - to protozoa (Protozoa, sporozoite Gregarinomorpha) parasitic on beetles). In conclusion, beetles are host species for bacteria, protozoa, fungi, nematodes, mites, Hymenoptera and Diptera. To complete data about parasite fauna beetles still will consult other papers from country and from abroad. Keywords: parasites, parasitoids, beetle-host. Rezumat. ContribuĠii la cunoaúterea cercetărilor privind parazitofauna la coleoptere (Insecta). Lucrarea prezintă o sinteză a datelor referitoare la parazitofauna unor specii de coleoptere preluate din lucrări publicate pentru România între 1959-2009 (CONSTANTINEANU, 1959; PANIN &SĂVULESCU, 1961; BALTHASAR, 1963; TUDOR, 1969; PISICĂ, 2001; PISICĂ &POPESCU, 2009) úi pentru străinătate între 1945-1966 (GYORFI, 1945-1947; BALACHOWSKY, 1962-1963; HURPIN 1962 pentru paraziĠi úi parazitoizi găsiĠi la specii de coleoptere precum úi GRASSE 1953 úi KUDO 1966, pentru protozoare parazite la diverse specii de coleoptere). În concluzie, gândaci sunt specii gazdă pentru bacterii, protozoare, ciuperci, nematode, acarieni, hymenoptere úi diptere. Pentru a avea date cât mai complete despre parazitofauna la coleoptere, în continuare vom consulta úi alte lucrări de specialitate din Ġarӽ cât úi din strӽinătate. -

The Geographic Variation of Saperda Inornata Say (Coleoptera: Cerambycidae) in Eastern North America
The Great Lakes Entomologist Volume 4 Number 2 -- Summer 1971 Number 2 -- Summer Article 2 1971 July 2017 The Geographic Variation of Saperda Inornata Say (Coleoptera: Cerambycidae) in Eastern North America John C. Nord USDA Forest Service, Athens, Georgia Fred B. Knight The University of Michigan, Ann Arbor Follow this and additional works at: https://scholar.valpo.edu/tgle Part of the Entomology Commons Recommended Citation Nord, John C. and Knight, Fred B. 2017. "The Geographic Variation of Saperda Inornata Say (Coleoptera: Cerambycidae) in Eastern North America," The Great Lakes Entomologist, vol 4 (2) Available at: https://scholar.valpo.edu/tgle/vol4/iss2/2 This Peer-Review Article is brought to you for free and open access by the Department of Biology at ValpoScholar. It has been accepted for inclusion in The Great Lakes Entomologist by an authorized administrator of ValpoScholar. For more information, please contact a ValpoScholar staff member at [email protected]. Nord and Knight: The Geographic Variation of Saperda Inornata Say (Coleoptera: Cer 1971 THE MICHIGAN ENTOMOLOGIST 39 THE GEOGRAPHIC VARIATION OF SAPERDA INORNA TA SAY (COLEOPTERA: CERAMBYCIDAE) IN EASTERN NORTH AMERICA John C. Nordl and Fred B. ~ni~ht~ During the summers of 1962 and 1963 a study of the life history and behavior of what was thought to have been Saperda moesta LeConte in trembling aspen, Populus tremuloides Michaux, was completed in northern Michigan (Nord, 1968). After the field study, it became apparent that the original identification was doubtful. Furthermore, there was a possibility that two species were present in the study areas, thus the biological data collected may have represented not one but two species. -

Management Indicator Species of the Kaibab National Forest: an Evaluation of Population and Habitat Trends Version 3.0 2010
Management Indicator Species of the Kaibab National Forest: an evaluation of population and habitat trends Version 3.0 2010 Isolated aspen stand. Photo by Heather McRae. Pygmy nuthatch. Photo by the Smithsonian Inst. Pumpkin Fire, Kaibab National Forest Mule deer. Photo by Bill Noble Red-naped sapsucker. Photo by the Smithsonian Inst. Northern Goshawk © Tom Munson Tree encroachment, Kaibab National Forest Prepared by: Valerie Stein Foster¹, Bill Noble², Kristin Bratland¹, and Roger Joos³ ¹Wildlife Biologist, Kaibab National Forest Supervisor’s Office ²Forest Biologist, Kaibab National Forest, Supervisor’s Office ³Wildlife Biologist, Kaibab National Forest, Williams Ranger District Table of Contents 1. MANAGEMENT INDICATOR SPECIES ................................................................ 4 INTRODUCTION .......................................................................................................... 4 Regulatory Background ...................................................................................................... 8 Management Indicator Species Population Estimates ...................................................... 10 SPECIES ACCOUNTS ................................................................................................ 18 Aquatic Macroinvertebrates ...................................................................................... 18 Cinnamon Teal .......................................................................................................... 21 Northern Goshawk ...................................................................................................