REPORTS of the TIBOR T. POLGAR FELLOWSHIP PROGRAM, 2013 David J. Yozzo, Sarah H. Fernald and Helena Andreyko Editors a Joint
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Greene County Open Space and Recreation Plan
GREENE COUNTY OPEN SPACE AND RECREATION PLAN PHASE I INVENTORY, DATA COLLECTION, SURVEY AND PUBLIC COMMENT DECEMBER 2002 A Publication of the Greene County Planning Department Funded in Part by a West of Hudson Master Planning and Zoning Incentive Award From the New York State Department of State Greene County Planning Department 909 Greene County Office Building, Cairo, New York 12413-9509 Phone: (518) 622-3251 Fax: (518) 622-9437 E-mail: [email protected] GREENE COUNTY OPEN SPACE AND RECREATION PLAN - PHASE I INVENTORY, DATA COLLECTION, SURVEY AND PUBLIC COMMENT TABLE OF CONTENTS I. Introduction ………………………………………………………………………………………………………………………………… 1 II. Natural Resources ……………………………………………………………………………………………………………………… 2 A. Bedrock Geology ………………………………………………………………………………………………………………… 2 1. Geological History ………………………………………………………………………………………………………… 2 2. Overburden …………………………………………………………………………………………………………………… 4 3. Major Bedrock Groups …………………………………………………………………………………………………… 5 B. Soils ……………………………………………………………………………………………………………………………………… 5 1. Soil Rating …………………………………………………………………………………………………………………… 7 2. Depth to Bedrock ………………………………………………………………………………………………………… 7 3. Suitability for Septic Systems ……………………………………………………………………………………… 8 4. Limitations to Community Development ………………………………………………………………… 8 C. Topography …………………………………………………………………………………………………………………………… 9 D. Slope …………………………………………………………………………………………………………………………………… 10 E. Erosion and Sedimentation ………………………………………………………………………………………………… 11 F. Aquifers ……………………………………………………………………………………………………………………………… -

Current Assessment of Fish Passage Opportunities in the Tributaries of the Lower Hudson River Carl W
Current Assessment of Fish Passage Opportunities in the Tributaries of the Lower Hudson River Carl W. Alderson1, Lisa Rosman2 1 NOAA Restoration Center, Highlands New Jersey, 2 NOAA-ORR/Assessment and Restoration Division, New York, New York NOAA’s Hudson River Fish Passage Initiative Study Team has identified 307 Lower Hudson Tributary Barrier Statistics Abstract barriers (r e d dots) to fish passage within the 65 major tributaries to the Lower • 307 Barriers Identified on 65 Tributaries (215 miles) Google Earth Elevation Profile Tool Hudson Estuary. Take notice of how The Hudson River estuary supports numerous diadromous and tightly these are clustered along the • 153 Dams, 23 Culverts/Bridges, 122 Natural, 9 TBD Demonstrating Three Examples of Potential Hudson Main Stem. Whether by the hand • Dams Constructed 1800-1999 potamodromous fish. Tributaries to the Hudson River provide critical of man or by nature’s rock, the first barrier Stream Miles Gained with Dam Removal spawning, nursery and foraging habitat for these migratory fish. to every tributary falls within short distance • Dam Height Range of 1 ft to 141 feet of the confluence of the Hudson. Here the Previous studies made recommendations for fish passage and were barriers are shown relative to the 5 major • Dam Length Range of 6 ft to 1,218 ft limited to determining the upstream fish movement at the first and watersheds of Lower Hudson from the • Spillway Width Range of 6 ft to 950 ft Battery in Manhattan to Troy, NY second barriers on each of 62 tributaries to the tidal (Lower) Hudson • Includes stream segments where slopes exceed 1:40 7 TODAY River (e.g., dams, culverts, natural falls/rapids) or to multiple barriers FUTURE GOAL Removal of dam 5 may allow • 73 Tributary Miles Currently Estimated Available to Diadromous Fish w/ dam removal 5 FUTURE GOAL EEL 6 eel to pass to RM 9.9 where for a small subset of tributaries. -

CHAINING the HUDSON the Fight for the River in the American Revolution
CHAINING THE HUDSON The fight for the river in the American Revolution COLN DI Chaining the Hudson Relic of the Great Chain, 1863. Look back into History & you 11 find the Newe improvers in the art of War has allways had the advantage of their Enemys. —Captain Daniel Joy to the Pennsylvania Committee of Safety, January 16, 1776 Preserve the Materials necessary to a particular and clear History of the American Revolution. They will yield uncommon Entertainment to the inquisitive and curious, and at the same time afford the most useful! and important Lessons not only to our own posterity, but to all succeeding Generations. Governor John Hancock to the Massachusetts House of Representatives, September 28, 1781. Chaining the Hudson The Fight for the River in the American Revolution LINCOLN DIAMANT Fordham University Press New York Copyright © 2004 Fordham University Press All rights reserved. No part of this publication may be reproduced, stored ii retrieval system, or transmitted in any form or by any means—electronic, mechanical, photocopy, recording, or any other—except for brief quotation: printed reviews, without the prior permission of the publisher. ISBN 0-8232-2339-6 Library of Congress Cataloging-in-Publication Data Diamant, Lincoln. Chaining the Hudson : the fight for the river in the American Revolution / Lincoln Diamant.—Fordham University Press ed. p. cm. Originally published: New York : Carol Pub. Group, 1994. Includes bibliographical references and index. ISBN 0-8232-2339-6 (pbk.) 1. New York (State)—History—Revolution, 1775-1783—Campaigns. 2. United States—History—Revolution, 1775-1783—Campaigns. 3. Hudson River Valley (N.Y. -
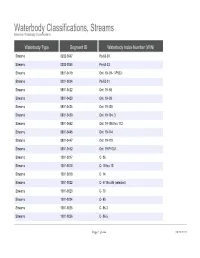
Waterbody Classifications, Streams Based on Waterbody Classifications
Waterbody Classifications, Streams Based on Waterbody Classifications Waterbody Type Segment ID Waterbody Index Number (WIN) Streams 0202-0047 Pa-63-30 Streams 0202-0048 Pa-63-33 Streams 0801-0419 Ont 19- 94- 1-P922- Streams 0201-0034 Pa-53-21 Streams 0801-0422 Ont 19- 98 Streams 0801-0423 Ont 19- 99 Streams 0801-0424 Ont 19-103 Streams 0801-0429 Ont 19-104- 3 Streams 0801-0442 Ont 19-105 thru 112 Streams 0801-0445 Ont 19-114 Streams 0801-0447 Ont 19-119 Streams 0801-0452 Ont 19-P1007- Streams 1001-0017 C- 86 Streams 1001-0018 C- 5 thru 13 Streams 1001-0019 C- 14 Streams 1001-0022 C- 57 thru 95 (selected) Streams 1001-0023 C- 73 Streams 1001-0024 C- 80 Streams 1001-0025 C- 86-3 Streams 1001-0026 C- 86-5 Page 1 of 464 09/28/2021 Waterbody Classifications, Streams Based on Waterbody Classifications Name Description Clear Creek and tribs entire stream and tribs Mud Creek and tribs entire stream and tribs Tribs to Long Lake total length of all tribs to lake Little Valley Creek, Upper, and tribs stream and tribs, above Elkdale Kents Creek and tribs entire stream and tribs Crystal Creek, Upper, and tribs stream and tribs, above Forestport Alder Creek and tribs entire stream and tribs Bear Creek and tribs entire stream and tribs Minor Tribs to Kayuta Lake total length of select tribs to the lake Little Black Creek, Upper, and tribs stream and tribs, above Wheelertown Twin Lakes Stream and tribs entire stream and tribs Tribs to North Lake total length of all tribs to lake Mill Brook and minor tribs entire stream and selected tribs Riley Brook -

Set in Scotland a Film Fan's Odyssey
Set in Scotland A Film Fan’s Odyssey visitscotland.com Cover Image: Daniel Craig as James Bond 007 in Skyfall, filmed in Glen Coe. Picture: United Archives/TopFoto This page: Eilean Donan Castle Contents 01 * >> Foreword 02-03 A Aberdeen & Aberdeenshire 04-07 B Argyll & The Isles 08-11 C Ayrshire & Arran 12-15 D Dumfries & Galloway 16-19 E Dundee & Angus 20-23 F Edinburgh & The Lothians 24-27 G Glasgow & The Clyde Valley 28-31 H The Highlands & Skye 32-35 I The Kingdom of Fife 36-39 J Orkney 40-43 K The Outer Hebrides 44-47 L Perthshire 48-51 M Scottish Borders 52-55 N Shetland 56-59 O Stirling, Loch Lomond, The Trossachs & Forth Valley 60-63 Hooray for Bollywood 64-65 Licensed to Thrill 66-67 Locations Guide 68-69 Set in Scotland Christopher Lambert in Highlander. Picture: Studiocanal 03 Foreword 03 >> In a 2015 online poll by USA Today, Scotland was voted the world’s Best Cinematic Destination. And it’s easy to see why. Films from all around the world have been shot in Scotland. Its rich array of film locations include ancient mountain ranges, mysterious stone circles, lush green glens, deep lochs, castles, stately homes, and vibrant cities complete with festivals, bustling streets and colourful night life. Little wonder the country has attracted filmmakers and cinemagoers since the movies began. This guide provides an introduction to just some of the many Scottish locations seen on the silver screen. The Inaccessible Pinnacle. Numerous Holy Grail to Stardust, The Dark Knight Scottish stars have twinkled in Hollywood’s Rises, Prometheus, Cloud Atlas, World firmament, from Sean Connery to War Z and Brave, various hidden gems Tilda Swinton and Ewan McGregor. -

Hillsdale, a History by Herbert S
Hillsdale, A History by Herbert S. Parmet Hillsdale Town Historian At the time of the American Revolution, the Taconic hill country was still basically an “unbroken wilderness,” in the words of a standard history of New York State. Most of what is now the Town of Hillsdale belonged to the Van Rensselaer family, major beneficiaries of the Dutch patroon system, and much of the modern town was within the manor of Rensselaerwyck. Colonial maps show that “Renslaerwick” also included Nobletown and Spencertown. The present location of Route 23 follows the line separating Rensselaer lands from Livingston Manor. Baronial farmlands were leased to proprietors and rented to tenant farmers. Much of upstate New York, including the vast Upper Manor of the Livingstons on the west side of the Hudson, resembled the old European feudal system. With the Revolutionary War, that system was ready for change. The Hudson Valley was one of the chief cradles of American independence, especially with the major battles fought around Saratoga and Ticonderoga. Much closer to the present Hillsdale, it furnished a route for one of the more heroic achievements of the battle for independence. Colonel Henry Knox, then only 25 and a former bookseller from Boston, joined with his brother in volunteering to retrieve munitions and cannon that had been captured by Ethan Allen and, improbable as it now seems, transported them over the snowy countryside to provide vital assistance for General Washington’s troops near Boston. The junction of county road 21 and Route 22, the site of the original Nobletown (the former name of Hillsdale), is a good place from which to appreciate Col. -

Designated Protected and Significant Areas of Dutchess County, NY
Chapter 7: Designated Significant and Protected Areas of Dutchess County (DRAFT) Chapter 7: Designated Protected and Significant Areas of Dutchess County, NY ______________________________________________________________________________ Emily Vail, Neil Curri, Noela Hooper, and Allison Chatrchyan1 February 2012 (DRAFT ) Significant natural areas are valued for their environmental importance Chapter Contents and beauty, and include unusual geologic features such as scenic Protected Land Critical Environmental mountain ridges, steep ravines, and caves; hydrological features such Areas as rivers, lakes, springs, and wetlands; and areas that support Other Significant Areas threatened or endangered species or unusually diverse plant and Implications for Decision- Making animal communities. Both significant natural areas and scenic Resources resources enhance the environmental health and quality of life in Dutchess County. An area can be significant for several different reasons, including its habitat, scenic, cultural, economic, or historical values. Many areas are significant because they are unique in some way. 1 This chapter was written by Emily Vail (Cornell Cooperative Extension Environment & Energy Program), Neil Curri (Cornell Cooperative Extension Dutchess County Environment & Energy Program), Noela Hooper (Dutchess County Department of Planning and Development), and Allison Chatrchyan (Cornell Cooperative Extension Dutchess County Environment & Energy Program). The chapter is presented here in DRAFT form. Final version expected March 2012. The Natural Resource Inventory of Dutchess County, NY 1 Chapter 7: Designated Significant and Protected Areas of Dutchess County (DRAFT) Significant natural areas provide many ecosystem services, including wildlife habitat, water supply protection, recreational space, and opportunities for outdoor research. (For more information on ecosystem services, see Chapter 1: Introduction.) In order to sustain their value, it is import to protect these areas. -

ICE Newsroom ICE Arrests More Than 80 During 5-Day Enforcement Action in New York City, the Hudson Valley, and Long Island
9/26/2019 ICE arrests more than 80 during 5-day enforcement action in New York City, the Hudson Valley, and Long Island | ICE Official Website of the Department of Homeland Security Report Crimes: Email or Call 1-866-DHS-2-ICE ICE Newsroom News Releases News Releases Enforcement and Removal 09/26/2019 Share ICE arrests more than 80 during 5-day enforcement action in New York City, the Hudson Valley, and Long Island NEW YORK – Officers from U.S. Immigration and Customs Enforcement's (ICE) Enforcement and Removal Operations (ERO) arrested more than 80 during a 5-day period, ending Sep. 25, in New York City, the Hudson Valley and Long Island. During the enforcement effort, ERO arrested 82 individuals for violating U.S. immigration laws. Of those arrested, more than 40 had been previously released from local law enforcement custody with an active detainer. Ten of those arrested were previously removed from the United States and returned illegally. Several had prior felony convictions for serious or violent offenses, such as sexual crimes, weapons charges, and assault, or had past convictions for significant or multiple misdemeanors. “Of those arrested during these five days, more than half were released from local custody with an active detainer. That is more than three dozen illegal aliens with convictions or criminal charges who were released back into New York communities, further putting the safety of its residents at risk,” said Thomas R. Decker, field office director for ERO New York. “Because of the senseless local laws in New York City, thousands of illegal aliens have been released; and as this trend continues, thousands more will be released back into the communities to threaten the safety of our city’s AILA Doc. -

The Kingbird Vol. 68 No. 4 – December 2018
New York State Ornithological Association, Inc. Vol. 68 No. 4 December 2018 Volume 68 No. 4 December 2018 pp. 257-360 CONTENTS Report of the New York State Avian Records Committee for 2014 . 258 New York State Ornithological Association, Inc., 71st Annual Meeting, Henrietta, New York, 6 October 2018 . 279 Patch Birding—The Stewart School Sump Brendan Fogarty . 286 Highlights of the Season, Summer 2018 Mike Cooper . 287 Regional Reports, Summer 2018 . 291 Photo Gallery, Summer 2018 . 307 Standard Regional Report Abbreviations, Reporting Deadlines and Map of Reporting Regions . 359 Editor – S. S. Mitra Regional Reports Editor – Robert G. Spahn Production Manager – Patricia J. Lindsay Circulation and Membership Manager – Patricia Aitken Front Cover: Eastern Kingbird, Tifft Nature Preserve, Erie, 29 Jun 2018, © Sue Barth. Back Cover: Eastern Kingbird, Birdsong Parklands, Erie, 11 Jul 2018, © Sue Barth. The Kingbird 2018 December; 68 (4) 257 REPORT OF THE NEW YORK STATE AVIAN RECORDS COMMITTEE FOR 2014 The New York State Avian Records Committee (hereafter “NYSARC” or the “Committee”) evaluated 123 submissions involving 69 occurrences of New York State review species from 2014, including 30 submissions involving four occurrences of potential first State records. Additionally, the Committee evaluated 4 submissions involving four occurrences of New York State review species from previous years. Reports were received from across the state, with 25 of the 62 counties represented plus the pelagic zone. The number of reports accompanied by photographs remains high and naturally benefits the value of the archive. The Committee wishes to remind readers that reports submitted to eBird, listservs, local bird clubs, rare bird alerts (RBAs) and even The Kingbird Regional Editors are not necessarily passed along to NYSARC. -

Greene County Grassland Habitat Management Plan
Greene County Grassland Habitat Management Plan Primary Authors: Karen Strong, Rene VanSchaack, and Ingrid Haeckel Contributing Authors: Abbe Martin (GCSWD) and Paul Novak Extra thanks for extensive comments and background material: Nancy Heaslip, Elizabeth LoGuidice, and Leslie Zucker. The Greene County Soil and Water Conservation District initiated the development of this management plan; however, a wide range of organizations and individuals played key roles in its development, and still more will have a role in implementation. Major project guidance was provided by the Greene County Habitat Advisory Committee, a unique partnership created to advise the Conservation District in habitat conservation. The following organizations and individuals are represented on the Greene County Habitat Advisory Committee and assisted in the development of this plan: The Greene County Habitat Advisory Committee Greene Land Trust: Bob Knighton Greene Industrial Development Agency: Rene VanSchaack Greene County Soil and Water Conservation District: Jeff Flack New York State Department of Environmental Conservation (NYSDEC): Paul Novak, Nancy Heaslip NYSDEC Hudson River Estuary Program: Karen Strong, Ingrid Haeckel Northern Catskills Audubon Society, Inc.: Larry Federman Scenic Hudson: Mark Wildonger, Chris Kenyon Hudsonia, Ltd.: Erik Kiviat, PhD. Cornell Cooperative Extension of Greene County’s Agroforestry Resource Center: Elizabeth LoGiudice Sierra Club, Hudson-Mohawk Group: Roger Downs Coxsackie Planning Board: Frank Gerrain Peter Feinberg, environmental consultant James Coe, artist, naturalist and field guide author Rich Guthrie, local bird expert New York State Department of Environmental Conservation provided funding for this project from the Environmental Protection Fund through the Hudson River Estuary Program. Suggested Citation: Strong, K., R. VanSchaack, and I. Haeckel. -

Roeliff Jansen Kill 2016
COMMUNITY WATER QUALITY MONITORING RESULTS ROELIFF JANSEN KILL 2016 OVERVIEW WATERSHED SNAPSHOT Riverkeeper and our partners have been testing These results are for non‐tidal sites only. the Hudson River and its tributaries for the As measured against the Environmental Protection fecal-indicator bacteria Enterococcus (“Entero”) Agency’s recommended Beach Action Value for safe since 2006. Sources of fecal contamination may swimming: include sewage infrastructure failures, sewer overflows, inadequate sewage treatment, septic system failures, agricultural runoff, urban 12% runoff, and wildlife. of Roeliff Jansen Kill samples failed. As measured against the EPA’s recommended In 2016, we began working with the Roe Jan geometric mean (GM, a weighted long‐term average) Watershed Community criterion for safe swimming: (www.roejanwatershed.org) and the Bard Water Lab to conduct testing in the Roeliff Jansen Kill. EPA GM threshold Roe Jan GM Samples were collected monthly (May to October) at 14 watershed locations by Roe Jan 30 24.8 Watershed Community members and local cells/100 mL cells/100 mL residents, and processed by the Bard Water Lab. A total of 84 samples were analyzed in 2016. This water quality monitoring study is designed 3 Best Sites 3 Worst Sites to learn about broad patterns and trends. The Livingston‐ Ancram‐ Hall Hill data can help inform choices about recreation in Above/Below Road Bridge (#6) the creek, but cannot predict future water Bingham Mills (#8, 9) Copake‐ Noster Kill quality at any particular time and place. Germantown‐ trib at Route 7A (#4) Sportsmen’s Club Ancram‐ Wiltsie All results will be available at (#10) Road Bridge fishing www.riverkeeper.org/water-quality/citizen- Hillsdale‐ Collins St access (#5) data/roeliff-jansen-kill soon. -

Before Albany
Before Albany THE UNIVERSITY OF THE STATE OF NEW YORK Regents of the University ROBERT M. BENNETT, Chancellor, B.A., M.S. ...................................................... Tonawanda MERRYL H. TISCH, Vice Chancellor, B.A., M.A. Ed.D. ........................................ New York SAUL B. COHEN, B.A., M.A., Ph.D. ................................................................... New Rochelle JAMES C. DAWSON, A.A., B.A., M.S., Ph.D. ....................................................... Peru ANTHONY S. BOTTAR, B.A., J.D. ......................................................................... Syracuse GERALDINE D. CHAPEY, B.A., M.A., Ed.D. ......................................................... Belle Harbor ARNOLD B. GARDNER, B.A., LL.B. ...................................................................... Buffalo HARRY PHILLIPS, 3rd, B.A., M.S.F.S. ................................................................... Hartsdale JOSEPH E. BOWMAN,JR., B.A., M.L.S., M.A., M.Ed., Ed.D. ................................ Albany JAMES R. TALLON,JR., B.A., M.A. ...................................................................... Binghamton MILTON L. COFIELD, B.S., M.B.A., Ph.D. ........................................................... Rochester ROGER B. TILLES, B.A., J.D. ............................................................................... Great Neck KAREN BROOKS HOPKINS, B.A., M.F.A. ............................................................... Brooklyn NATALIE M. GOMEZ-VELEZ, B.A., J.D. ...............................................................