Acute Neurological Findings in a National Cohort of Neonates With
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Neonatal Orthopaedics
NEONATAL ORTHOPAEDICS NEONATAL ORTHOPAEDICS Second Edition N De Mazumder MBBS MS Ex-Professor and Head Department of Orthopaedics Ramakrishna Mission Seva Pratishthan Vivekananda Institute of Medical Sciences Kolkata, West Bengal, India Visiting Surgeon Department of Orthopaedics Chittaranjan Sishu Sadan Kolkata, West Bengal, India Ex-President West Bengal Orthopaedic Association (A Chapter of Indian Orthopaedic Association) Kolkata, West Bengal, India Consultant Orthopaedic Surgeon Park Children’s Centre Kolkata, West Bengal, India Foreword AK Das ® JAYPEE BROTHERS MEDICAL PUBLISHERS (P) LTD. New Delhi • London • Philadelphia • Panama (021)66485438 66485457 www.ketabpezeshki.com ® Jaypee Brothers Medical Publishers (P) Ltd. Headquarters Jaypee Brothers Medical Publishers (P) Ltd. 4838/24, Ansari Road, Daryaganj New Delhi 110 002, India Phone: +91-11-43574357 Fax: +91-11-43574314 Email: [email protected] Overseas Offices J.P. Medical Ltd. Jaypee-Highlights Medical Publishers Inc. Jaypee Brothers Medical Publishers Ltd. 83, Victoria Street, London City of Knowledge, Bld. 237, Clayton The Bourse SW1H 0HW (UK) Panama City, Panama 111, South Independence Mall East Phone: +44-2031708910 Phone: +507-301-0496 Suite 835, Philadelphia, PA 19106, USA Fax: +02-03-0086180 Fax: +507-301-0499 Phone: +267-519-9789 Email: [email protected] Email: [email protected] Email: [email protected] Jaypee Brothers Medical Publishers (P) Ltd. Jaypee Brothers Medical Publishers (P) Ltd. 17/1-B, Babar Road, Block-B, Shaymali Shorakhute, Kathmandu Mohammadpur, Dhaka-1207 Nepal Bangladesh Phone: +00977-9841528578 Mobile: +08801912003485 Email: [email protected] Email: [email protected] Website: www.jaypeebrothers.com Website: www.jaypeedigital.com © 2013, Jaypee Brothers Medical Publishers All rights reserved. No part of this book may be reproduced in any form or by any means without the prior permission of the publisher. -

Necrotizing Enterocolitis in a Newborn Following Intravenous Immunoglobulin Treatment for Haemolytic Disease
CASE REPORT Necrotizing Enterocolitis in a Newborn Following Intravenous Immunoglobulin Treatment for Haemolytic Disease Semra Kara1, Hulya Ulu-ozkan2, Yavuz Yilmaz2, Fatma Inci Arikan3, Ugur Dilmen4 and Yildiz Dallar Bilge3 ABSTRACT ABO iso-immunization is the most frequent haemolytic disease of the newborn. Treatment depends on the total serum bilirubin level, which may increase very rapidly in the first 48 hours of life in cases of haemolytic disease of the newborn. Phototherapy and, in severe cases, exchange transfusion are used to prevent hyperbilirubinaemic encephalopathy. Intravenous immunoglobulins (IVIG) are used to reduce exchange transfusion. Herein, we present a female newborn who was admitted to the NICU because of ABO immune haemolytic disease. After two courses of 1 g/kg of IVIG infusion, she developed necrotizing enterocolitis (NEC). Administration of IVIG to newborns with significant hyperbilirubinaemia due to ABO haemolytic disease should be cautiously administered and followed for complications. Key Words: Necrotizing enterocolitis. Hyperbilirubinaemia. Newborn. Intravenous immunoglobulins. Iso-immunization. INTRODUCTION important adverse reactions,6 one of which is described Blood group incompatibilities are most frequent and hereby. severe conditions causing hyperbilirubinaemia in the neonatal period.1 Phototherapy and in severe cases CASE REPORT exchange transfusion are used to prevent kernicterus A female newborn was admitted to our neonatal unit and reduce perinatal mortality. Exchange transfusion because of jaundice on the tenth hours after being born. is not free of severe complications such as thrombo- The baby was born by uncomplicated vaginal delivery to cytopenia, apnoea, pulmonary haemorrhage, haemodyna- a healthy 31-year-old mother who was fourth gravida mic instability, septicaemia and necrotizing enterocolitis and second para. -

Hypotonia and Lethargy in a Two-Day-Old Male Infant Adrienne H
Hypotonia and Lethargy in a Two-Day-Old Male Infant Adrienne H. Long, MD, PhD,a,b Jennifer G. Fiore, MD,a,b Riaz Gillani, MD,a,b Laurie M. Douglass, MD,c Alan M. Fujii, MD,d Jodi D. Hoffman, MDe A 2-day old term male infant was found to be hypotonic and minimally abstract reactive during routine nursing care in the newborn nursery. At 40 hours of life, he was hypoglycemic and had intermittent desaturations to 70%. His mother had an unremarkable pregnancy and spontaneous vaginal delivery. The mother’s prenatal serology results were negative for infectious risk factors. Apgar scores were 9 at 1 and 5 minutes of life. On day 1 of life, he fed, stooled, and voided well. Our expert panel discusses the differential diagnosis of hypotonia in a neonate, offers diagnostic and management recommendations, and discusses the final diagnosis. DRS LONG, FIORE, AND GILLANI, birth weight was 3.4 kg (56th PEDIATRIC RESIDENTS percentile), length was 52 cm (87th aDepartment of Medicine, Boston Children’s Hospital, d e percentile), and head circumference Boston, Massachusetts; and Neonatology Section, Medical A 2-day old male infant born at Genetics Section, cDivision of Child Neurology, and 38 weeks and 4 days was found to be was 33 cm (12th percentile). His bDepartment of Pediatrics, Boston Medical Center, Boston, limp and minimally reactive during physical examination at birth was Massachusetts routine care in the newborn nursery. normal for gestational age, with Drs Long, Fiore, and Gillani conceptualized, drafted, Just 5 hours before, he had an appropriate neurologic, cardiac, and and edited the manuscript; Drs Douglass, Fujii, and appropriate neurologic status when respiratory components. -

Hypotonia Surestep Product Catalog Page 29 in Step with Pediatric Hypotonia
SPECIAL EDUCATIONAL SERIES DIAGNOSTIC INSIGHTS ANALYZING GAIT CHANGES GROSS MOTOR SKILLS ORTHOTIC MANAGEMENT CLI N I CAL CASE STUDIES Sponsored by an educational grant from: In Step With Pediatric Hypotonia SureStep Product Catalog Page 29 In Step With Pediatric Hypotonia Contents VIEWPOINT FROM THE EDITOR: An Unexpected Path, Mobility and More an Invaluable Perspective At the most basic level, mobility is about get- PAGE 3 ting from point A to point B. But, for many children with hypotonia, it’s about so much 4 more. FEATURES It’s about independence. It’s about con- fidence. It’s about maintaining strength, fit- ness, and healthy bones. It’s about not being Understanding Hypotonia excluded from activities enjoyed by their PAGE 4 typically developing peers. And improved mobility may have even Gait: The Cornerstone more benefits in those children whose hy- potonia is associated with social and behav- of Intervention ioral developmental delays. New research PAGE 8 has identified an association between motor skills and sociobehavioral milestones in chil- 8 The Importance of Gross dren with autism spectrum disorder, who often present with hypotonia (see “The Im- Motor Skills portance of Gross Motor Skills,” page 12). PAGE 12 This suggests that early intervention to improve gross motor skills—including or- thotic devices and physical therapy—may Orthotic Solutions for also help certain children interact more Children with Hypotonia comfortably with others. That won’t come as PAGE 16 a surprise to the clinicians and parents who 12 have personally seen it happen. This special issue is filled with evidence- Orthotic Success Stories: based information and personal success sto- Four Cases in a Series ries illustrating how effective interventions can enhance mobility in children with hy- PAGE 20 potonia. -

Hyperbilirubinemia and Neonatal Infection
Original Article International Journal of Pediatrics, Vol.1, Serial No.1, Dec 2013 Hyperbilirubinemia and Neonatal Infection Gholamali Maamouri1, Fatemah Khatami1, Ashraf Mohammadzadeh1, Reza saeidi1, Ahmad shah Farhat1, Mohammad Ali Kiani1,*Hassan Boskabadi1 1Neonatal Research Center , Mashhad University of Medical Science (MUMS), Mashhad, Iran. Abstract Introduction: Hyperbilirubinemia is a relatively common disorder among infants in Iran. Bacterial infection and jaundice may be associated with higher morbidity. Previous studies have reported that jaundice may be one of the signs of infection. The aim of this study was to determine the incidence rate, presentation time, severity of jaundice, signs and complications of infection within neonatal hyperbilirubinemia. Materials and Methods: This cross sectional study was conducted between 2003 and 2011, at Ghaem Hospital, Mashhad- Iran. We prospectively evaluated 1763 jaundiced newborns. We finally found 434 neonates who were categorized into two groups.131 neonates as case group (Blood or/and Urine culture positive or sign of pneumonia) and 303 neonates with idiopathic jaundice as control group. Demographic data including prenatal, intrapartum, postnatal events and risk factors were collected by questionnaire. Biochemical markers including bilirubin level, urine and blood cultures were determined at the request of the clinicians. Results: Jaundice presentation time, age on admission, serum bilirubin value and hospitalization period were reported significantly higher among case group in comparison with control group (p<0.0001). Urinary tract infection (UTI), sepsis and pneumonia were detected in 102 (8%), 22 (1.7%) and 7 (0.03%) cases, respectively. Conclusion: We concluded that bacterial infection was a significant cause of unexplained Hyperbilirubinemia among jaundice newborns (10%). -

Delayed Cord Clamping in Red Blood Cell Alloimmunization: Safe, Effective, and Free?
Editorial Delayed cord clamping in red blood cell alloimmunization: safe, effective, and free? Ryan M. McAdams Department of Pediatrics, University of Washington, Seattle, WA, USA Correspondence to: Ryan M. McAdams, MD, Associate Professor. Division of Neonatology, Department of Pediatrics, University of Washington, Box 356320, Seattle, WA 98195-6320, USA. Email: [email protected]. Abstract: Hemolytic disease of the newborn (HDN), an alloimmune disorder due to maternal and fetal blood type incompatibility, is associated with fetal and neonatal complications related to red blood cell (RBC) hemolysis. After delivery, without placental clearance, neonatal hyperbilirubinemia may develop from ongoing maternal antibody-mediated RBC hemolysis. In cases refractory to intensive phototherapy treatment, exchange transfusions (ET) may be performed to prevent central nervous system damage by reducing circulating bilirubin levels and to replace antibody-coated red blood cells with antigen-negative RBCs. The risks and costs of treating HDN are significant, but appear to be decreased by delayed umbilical cord clamping at birth, a strategy that promotes placental transfusion to the newborn. Compared to immediate cord clamping (ICC), safe and beneficial short-term outcomes have been demonstrated in preterm and term neonates receiving delayed cord clamping (DCC), a practice that may potentially be effective in cases RBC alloimmunization. Keywords: Red blood cell (RBC); delayed cord clamping (DCC); exchange transfusions (ET) Submitted Mar 29, 2016. Accepted for publication Apr 11, 2016. doi: 10.21037/tp.2016.04.02 View this article at: http://dx.doi.org/10.21037/tp.2016.04.02 Hemolytic disease of the newborn (HDN) is an alloimmune exchange transfusions (ET) may be performed to prevent disorder caused by transplacentally transmitted maternal kernicterus by reducing circulating bilirubin levels and immunoglobulin (Ig) G antibodies that bind to paternally to replace antibody-coated RBCs with antigen-negative inherited antigens present on fetal red blood cells RBCs. -

JMSCR Vol||05||Issue||03||Page 19659-19665||March 2017
JMSCR Vol||05||Issue||03||Page 19659-19665||March 2017 www.jmscr.igmpublication.org Impact Factor 5.84 Index Copernicus Value: 83.27 ISSN (e)-2347-176x ISSN (p) 2455-0450 DOI: https://dx.doi.org/10.18535/jmscr/v5i3.210 Maternal and Neonatal Determinants of Neonatal Jaundice – A Case Control Study Authors Sudha Menon1, Nadia Amanullah2 1Additional Professor, Department of Obstetrics and Gynecology, Government Medical College Trivandrum, Kerala, South India 2Msc Nursing Student, Government Nursing College, Trivandrum Corresponding Author Dr Sudha Menon Email: [email protected] ABSTRACT Neonatal jaundice a common condition which affects about 60-80% of newborn and if severe can lead serious neurological sequelae. Determinants include neonatal and maternal factors. A prospective case control descriptive study in a tertiary care centre was done in 62 consecutive cases with neonatal jaundice and 124 consecutive newborns without jaundice served as controls. The major determinants for neonatal jaundice were low birth weight <2.5 kg (OR 24.54 95% CI 10.98 – 54.84 : P<0.0001), birth asphyxia (OR 16.5; 95% CI 4.63- 58.76, P<0.0001), Low APGAR score <7( OR 26.1; 95% CI 3.28- 207.47, P =0.0002), prematurity <37weeks ( OR 28.92 95% CI 12.15-68.82 P=0.0001) Preterm premature rupture of membranes (OR 58.57 95% CI 7.67 -449.87 P<0.0001) and malpresentation (OR 14.64 95% CI 3.16 - 67.792 tailed p =0.00004).The factors which evolved significant on logistic regression were Preterm premature rupture of membranes , gestational age <37 weeks , APGAR score below 7, Birth weight below 2500grams, Birth asphyxia and multiple pregnancy. -

Newborn Metabolic Profile Associated with Hyperbilirubinemia with and Without Kernicterus
Citation: Clin Transl Sci (2019) 12, 28–38; doi:10.1111/cts.12590 ARTICLE Newborn Metabolic Profile Associated with Hyperbilirubinemia With and Without Kernicterus Molly E. McCarthy1,2,*, Scott P. Oltman3, Rebecca J. Baer4,5, Kelli K. Ryckman6, Elizabeth E. Rogers7, Martina A. Steurer-Muller8, John S. Witte9 and Laura L. Jelliffe-Pawlowski10 Our objective was to assess the relationship between hyperbilirubinemia with and without kernicterus and metabolic profile at newborn screening. Included were 1,693,658 infants divided into a training or testing subset in a ratio of 3:1. Forty- two metabolites were analyzed using logistic regression (odds ratios (ORs), area under the receiver operating characteristic curve (AUC), 95% confidence intervals (CIs)). Several metabolite patterns remained consistent across gestational age groups for hyperbilirubinemia without kernicterus. Thyroid stimulating hormone (TSH) and C- 18:2 were decreased, whereas tyrosine and C- 3 were increased in infants across groupings. Increased C- 3 was also observed for kernicterus (OR: 3.17; 95% CI: 1.18–8.53). Thirty- one metabolites were associated with hyperbilirubinemia without kernicterus in the training set. Phenylalanine (OR: 1.91; 95% CI: 1.85–1.97), ornithine (OR: 0.76; 95% 0.74–0.77), and isoleucine + leucine (OR: 0.63; 95% CI: 0.61–0.65) were the most strongly associated. This study showed that newborn metabolic function is associated with hyper- bilirubinemia with and without kernicterus. Study Highlights WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC? WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE? ✔ A variety of neonatal and maternal characteristics are ✔ Forty-one infant metabolites collected at newborn known to be associated with increased risk of hyperbili- screening were shown to be associated with neonatal hy- rubinemia. -

The Effect of the Use of Oxytocin in Labor on Neonatal Jaundice
http:// ijp.mums.ac.ir Systematic Review (Pages: 6541-6553) The Effect of the Use of Oxytocin in Labor on Neonatal Jaundice: A Systematic Review and Meta-Analysis Robabe Seyedi1, *Mojgan Mirghafourvand 2, Shirin Osouli Tabrizi11 1Department of Midwifery, Students Research Committee, Faculty of Nursing and Midwifery, Tabriz University of Medical Sciences, Tabriz, Iran. 2Associate Professor, Social Determinants of Health Research Center, Tabriz University of Medical Sciences, Tabriz, Iran. Abstract Background: Neonatal Jaundice is a common problem that occurs in most preterm and term neonates. This systematic review aimed to examine the evidence for the effects of oxytocin in labor on neonatal jaundice. Materials and Methods: In this systematic review study, English databases including PubMed, Google Scholar, Embase, Cochrane Library, Scopus, Web of Sciences, and Persian databases including SID, Magiran, and Barakat Knowledge Network System Were searched from Jan 1980 to Dec 2017. Persian and English human clinical trials were targeted. The review was limited to human clinical trials examining the effect of oxytocin in labor on neonatal jaundice. The searched MESH vocabulary was "neonatal hyperbilirubinemia" OR "jaundice" in combination with "oxytocin in labor" OR "induction of labor". Two authors examined the articles separately and the disagreements were resolved through discussion. Results: Out of 583 articles searched in the databases, 440 title, 83 abstracts, and 60 full texts were reviewed, of which 5 English language articles entered the study and 4 articles entered the meta- analysis. Meta-analysis results showed that oxytocin did not affect the serum bilirubin level of the umbilical cord (Mean difference: 1.60; 95% confidence interval [CI]:-2.50 to 5.69; P=0.44; I2=78%). -
IUGR: Intrauterine Growth Restriction
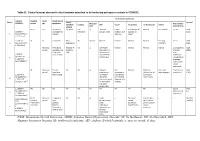
Table S1. Clinical features observed in the 6 patients described so far harboring pathogenic variants in FOXRED1. Evolutionary symptoms Variants Prenatal Onset Onset clinical Patient Lactic/ Survival FOXRED1 period age symptoms Muscular Psychomotor Metabolic Epilepsy MRI Visual Respiratory Cardiovascular Others tone development acidosis IUGR 2m Hypotonia Yes (+++) Yes ↓ Normal Latent Bronchospasm Normal AEP normal IQ: 48 Alive c.920G>A Development refractary (2m,4y,7y3m) strabismus of episodes in (15y) 1 (p.Gly307Glu) / al delay right eye infant c.733+1G>A c.920G>A NI 4y Clumsiness With No Normal Normal Normal Normal Normal Learning IQ: 99 Alive 2 (p.Gly307Glu) / exercise (+) disorders (19y) c.733+1G>A - Neonatal Premature; No (only ↑ Yes ↓ Decreased Normal Normal Normal Normal Gradually loss Alive period Hypoglycemia lactate in attenuation in of motor (22y)) Congenital LCR) the putamen skills; c.694C>T lactic acidosis and cerebellar wheelchair; (p.Gln232X) / 3 atrophy (6y) no expressive c.1289A>G language; 18 (p.Asn430Ser) understands simple commands NI Neonatal Truncal Yes Yes ↓ Delayed Eye Normal Mild non- Persistent Psychomotor Alive period hypotonia myelination movements obstructive left hepatomegaly retardation (10y) c.1054 C>T Poor feeding ventricular have always ventricular (p.Arg352Trp) / dilatation; been roving hypertrophy 4 c.1054 C>T abnormal signal bilateral optic (p.Arg352Trp) 19 in the thalami atrophy and basal ganglia (8m) c.1308G>A ND ND ND ND Yes NA NA NA NA NA NA Severe Alive (p.Val421Met) / psychomotor (¿) 5 c.1308G>A retardation (p.Val421Met)20 IUGR; Neonatal Congenital Yes Yes ↓ Large -- Persistent Dilated right - - Death (3 c.612_615dupA Cerebral period lactic periventricular severe ventricle and months) CTG intraventric acidosis. -

Cerebral Hypotonia by Mihee Bay MD (Dr
Cerebral hypotonia By Mihee Bay MD (Dr. Bay of Kennedy Krieger Institute and Johns Hopkins School of Medicine has no relevant financial relationships to disclose.) Originally released July 12, 2006; last updated February 1, 2016; expires February 1, 2019 Introduction This article includes discussion of cerebral hypotonia, central hypotonia, essential hypotonia, benign congenital hypotonia, and floppy infant. The foregoing terms may include synonyms, similar disorders, variations in usage, and abbreviations. Overview Hypotonia is a clinical manifestation of numerous diseases affecting the central and/or peripheral motor nervous system. The key to accurate diagnosis involves integral steps of evaluation that include a detailed history, examination, and diagnostic tests. “Cerebral” (or central) hypotonia implies pathogenesis from abnormalities from the central nervous system, and related causal disorders include cerebral dysgenesis and genetic or metabolic disorders. Patients with central hypotonia generally have hypotonia without associated weakness, in contrast to the peripheral (lower motor neuron) causes, which typically produce both hypotonia and muscle weakness. Hypotonia is a clinical manifestation of over 500 genetic disorders; thus, a logical, stepwise approach to diagnosis is essential. With recent advances in the field of genetic testing, diagnostic yield will undoubtedly improve. There is no cure, but treatment includes supportive therapies, such as physical and occupational therapy, and diagnosis-specific management. Key points • Hypotonia is reduced tension or resistance of passive range of motion. • The first step in the evaluation of a child with hypotonia is localization to the central (“cerebral”) or peripheral nervous system, or both. • Central hypotonia is more likely to be noted axially with normal strength and hyperactive to normal deep tendon reflexes. -

Hyperbilirubinemia and Kernicterus Jesus Peinado PGY2 Merle Ipson MD March 2009 Hyperbilirubinemia
Hyperbilirubinemia and Kernicterus Jesus Peinado PGY2 Merle Ipson MD March 2009 Hyperbilirubinemia Most common clinical condition requiring evaluation and treatment in the NB Most common cause of readmission in the 1 st week Generally a benign transitional phenomenon May pose a direct threat of brain damage May evolve into kernicterus Kernicterus 1. Choreoathetoid cerebral palsy 2. High-frequency central neural hearing loss 3. Palsy of vertical gaze 4. Dental enamel hypoplasia (result of bilirubin-induced cell toxicity) Kernicterus Originally described in NB with Rh hemolytic disease Recently reported in healthy term and late preterm Reported in breast-fed infants w/out hemolysis Most prevalent risk factor is late preterm Late Preterm Infant Relatively immature in their capacity to handle unconjugated bilirubin Hyperbilirubinemia is more prevalent, pronounced and protracted Eightfold increased risk of developing TSB > 20 mg/dl (5.2%) compared to term (0.7%) Pathobiology Increased bilirubin load in the hepatocyte Decreased erythrocyte survival Increased erythrocyte volume Increased enterohepatic circulation Decreased hepatic uptake from plasma Defective bilirubin conjugation Bilirubin Metabolism Bilirubin Metabolism Bilirubin Metabolism How bilirubin Damages the Brain Determinants of neuronal injury by bilirubin 1. Concentration of unconjugated bilirubin 2. Free bilirubin 3. Concentration of serum albumin 4. Ability to bind UCB 5. Concentration of hydrogen ion 6. Neuronal susceptibility Intracellular Calcium Homeostasis