Irregular Branching Douglas’ Neckera Neckera Douglasii Hanging Mats Arching out from Tree Trunks Or Branches
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
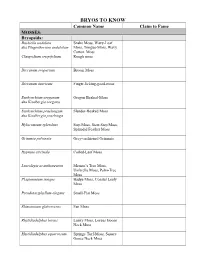
Bryo's to Know Table
BRYOS TO KNOW Common Name Claim to Fame MOSSES: Bryopsida: Buckiella undulata Snake Moss, Wavy-Leaf aka Plagiothecium undulatum Moss, Tongue-Moss, Wavy Cotton, Moss Claopodium crispifolium Rough moss Dicranum scoparium Broom Moss Dicranum tauricum Finger-licking-good-moss Eurhynchium oreganum Oregon Beaked-Moss aka Kindbergia oregana Eurhynchium praelongum Slender-Beaked Moss aka Kindbergia praelonga Hylocomium splendens Step Moss, Stair-Step Moss, Splendid Feather Moss Grimmia pulvinata Grey-cushioned Grimmia Hypnum circinale Coiled-Leaf Moss Leucolepis acanthoneuron Menzie’s Tree Moss, Umbrella Moss, Palm-Tree Moss Plagiomnium insigne Badge Moss, Coastal Leafy Moss Pseudotaxiphyllum elegans Small-Flat Moss Rhizomnium glabrescens Fan Moss Rhytidiadelphus loreus Lanky Moss, Loreus Goose Neck Moss Rhytidiadelphus squarrosum Springy Turf-Moss, Square Goose Neck Moss Rhytidiadelphus triquetrus Electrified Cat-Tail Moss, Goose Necked Moss Rhytidiopsus robusta Robust mountain moss Schistostega pennata Goblin’s Gold, Luminous Moss Polytrichopsida: Atrichum Atrichum Moss , Crane’s Bill Moss (for Atrichum selwynii) Pogonatum contortum Contorted Pogonatum Moss Polytrichum commune Common Hair Cap Moss Polytrichum piliferum Bristly Haircap Moss Andreaeopsida Andreaea nivalis Granite moss, Lantern moss, Snow Rock Moss Sphagnopsida: Sphagnum capillifolium Red Bog Moss, Small Red Peat Moss Sphagnum papillosum Fat Bog Moss, Papillose sphagnum Sphagnum squarrosum Shaggy Sphagnum, Spread- Leaved Peat Moss Takakiopsida: Takakia lepidoziooides Impossible -

Revision and Checklist of the Moss Families Bartramiaceae and Mniaceae in Vietnam Timo KOPONEN1, Thanh-Luc NGUYEN2, Thien-Tam L
Hattoria 10: 69–107. 2019 Revision and checklist of the moss families Bartramiaceae and Mniaceae in Vietnam Timo KOPONEN1, Thanh-Luc NGUYEN2, Thien-Tam LUONG3, 4 & Sanna HUTTUNEN4 1 Finnish-Chinese Botanical Foundation, Mailantie 109, FI-08800 Lohja, Finland & Finnish Museum of Natural History, Botany Unit (bryology), P.O. Box 7 (Unioninkatu 4), FI-00014 University of Helsinki, Finland 2 Southern Institute of Ecology, Vietnam Academy of Science and Technology, 1 Mac Dinh Chi, District 1, Ho Chi Minh City, Vietnam 3 University of Science, Vietnam National University Ho Chi Minh City, 227 Nguyen Van Cu, District 5, Ho Chi Minh City, Vietnam 4 Herbarium (TUR), Biodiversity Unit, FI 20014 University of Turku, Finland Author for correspondence: Thanh-Luc NGUYEN, [email protected] Abstract The genera Fleischerobryum Loeske and Philonotis Brid. of the Bartramiaceae and the family Mniaceae (excluding Pohlia Hedw.) are revised for Vietnam, based on specimens studied and literature reports. Four species are added to the flora: Orthomnion javense (M.Fleisch.) T.J.Kop., Philonotis asperifolia Mitt., P. laii T.J.Kop., P. speciosa (Griff.) Mitt. syn. nov. (based on P. mercieri Paris & Broth.), and Plagiomnium wui (T.J.Kop.) Y.J.Yi & S.He. Eight species are excluded from the flora. Two taxa are considered doubtful. The flora now includes one species of Fleischerobryum, eight species of Philonotis, one species of Mnium Hedw. (doubtful), three species of Orthomnion Wills. and five species of Plagiomnium (one doubtful). The 15 species are divided into phytogeographical elements. Eight belong to the Southeast Asiatic temperate to meridional element, and seven to the Southeast Asiatic meridional to subtropical element. -

Coptis Trifolia Conservation Assessment
CONSERVATION ASSESSMENT for Coptis trifolia (L.) Salisb. Originally issued as Management Recommendations December 1998 Marty Stein Reconfigured-January 2005 Tracy L. Fuentes USDA Forest Service Region 6 and USDI Bureau of Land Management, Oregon and Washington CONSERVATION ASSESSMENT FOR COPTIS TRIFOLIA Table of Contents Page List of Tables ................................................................................................................................. 2 List of Figures ................................................................................................................................ 2 Summary........................................................................................................................................ 4 I. NATURAL HISTORY............................................................................................................. 6 A. Taxonomy and Nomenclature.......................................................................................... 6 B. Species Description ........................................................................................................... 6 1. Morphology ................................................................................................................... 6 2. Reproductive Biology.................................................................................................... 7 3. Ecological Roles ............................................................................................................. 7 C. Range and Sites -

Riparian Bryophytes of the H. J. Andrews Experimental Forest in the Western Cascades, Oregon
The Bryologist 99(2), pp. 226-235 Copyright © 1996 by the American Bryological and Lichenological Society, Inc. Riparian Bryophytes of the H. J. Andrews Experimental Forest in the Western Cascades, Oregon BENGT GUNNAR JONSSON I Department of Forest Science, Oregon State University, Corvallis, OR 97331 Abstract. The knowledge of the distribution and habitat demands for bryophytes in the Pacific Northwest is scarce, and few published quantitative accounts of the flora are present. The present paper includes habitat description, elevational range, substrate preference, and frequency esti- mates for more than 130 riparian mosses and liverworts found in old-growth Pseudotsuga-Tsuga forests of the H. J. Andrews Experimental Forest, Oregon. The data are based on 360 samples distributed among 42 sites covering 1st to 5th order streams and 420 to 1250 m. TWINSPAN analysis resulted in 6 sample groups, representing samples from different elevations, geomorphic surfaces, and stream sizes. The most common mosses are Eurhynchium oreganum, Isothecium stoloniferum, Hypnum circinale, and Dicranum fuscescens. Among the hepatics Scapania bolan- deri, Cephalozia lunulifolia, and Porella navicularis are the most abundant species. Most species are rare at both site and sampte gtot (ever; and this is especially true for acrocarps where more than one-third of the observed species occurred in only one or two sites orland samples. Four of the occurring species (i.e., Antitrichia curtipendula, Buxbaumia piperi, Douinia ovata, and Ptili- dium californicum) are listed for special management and/or regional surveys. Bryophytes constitute an important and conspic- a wide range of different substrates, disturbance uous component of old-growth forests in the Pacific patterns, and a moist microclimate are important Northwest (Lesica et al. -

Monoicous Species Pairs in the Mniaceae (Bryophyta); Morphology, Sexual Condition and Distiribution
ISSN 2336-3193 Acta Mus. Siles. Sci. Natur., 68: 67-81, 2019 DOI: 10.2478/cszma-2019-0008 Published: online 1 July 2019, print July 2019 On the hypothesis of dioicous − monoicous species pairs in the Mniaceae (Bryophyta); morphology, sexual condition and distiribution Timo Koponen On the hypothesis of dioicous − monoicous species pairs in the Mniaceae (Bryophyta); morphology, sexual condition and distiribution. – Acta Mus. Siles. Sci. Natur., 68: 67-81, 2019. Abstract: Some early observations seemed to show that, in the Mniaceae, the doubling of the chromo- some set affects a change from dioicous to monoicous condition, larger size of the gametophyte including larger leaf cell size, and to a wider range of the monoicous counterpart. The Mniaceae taxa are divided into four groups based on their sexual condition and morphology. 1. Dioicous – monoicous counterparts which can be distinguished by morphological characters, 2. Dioicous – monoicous taxa which have no morphological, deviating characters, 3. Monoicous species mostly with diploid chromosome number for which no dioicous counterpart is known, and 4. The taxa in Mniaceae with only dioicous plants. Most of the monoicous species of the Mniaceae have wide ranges, but a few of them are endemics in geographically isolated areas. The dioicous species have either a wide holarctic range or a limited range in the forested areas of temperate and meridional North America, Europe and SE Asia, or in subtropical Asia. Some of the monoicous species are evidently autodiploids and a few of them are allopolyploids from cross-sections of two species. Quite recently, several new possible dioicous – monoicous relationships have been discovered. -

Flora of New Zealand Mosses
FLORA OF NEW ZEALAND MOSSES BRACHYTHECIACEAE A.J. FIFE Fascicle 46 – JUNE 2020 © Landcare Research New Zealand Limited 2020. Unless indicated otherwise for specific items, this copyright work is licensed under the Creative Commons Attribution 4.0 International licence Attribution if redistributing to the public without adaptation: "Source: Manaaki Whenua – Landcare Research" Attribution if making an adaptation or derivative work: "Sourced from Manaaki Whenua – Landcare Research" See Image Information for copyright and licence details for images. CATALOGUING IN PUBLICATION Fife, Allan J. (Allan James), 1951- Flora of New Zealand : mosses. Fascicle 46, Brachytheciaceae / Allan J. Fife. -- Lincoln, N.Z. : Manaaki Whenua Press, 2020. 1 online resource ISBN 978-0-947525-65-1 (pdf) ISBN 978-0-478-34747-0 (set) 1. Mosses -- New Zealand -- Identification. I. Title. II. Manaaki Whenua-Landcare Research New Zealand Ltd. UDC 582.345.16(931) DC 588.20993 DOI: 10.7931/w15y-gz43 This work should be cited as: Fife, A.J. 2020: Brachytheciaceae. In: Smissen, R.; Wilton, A.D. Flora of New Zealand – Mosses. Fascicle 46. Manaaki Whenua Press, Lincoln. http://dx.doi.org/10.7931/w15y-gz43 Date submitted: 9 May 2019 ; Date accepted: 15 Aug 2019 Cover image: Eurhynchium asperipes, habit with capsule, moist. Drawn by Rebecca Wagstaff from A.J. Fife 6828, CHR 449024. Contents Introduction..............................................................................................................................................1 Typification...............................................................................................................................................1 -

Heathland Wind Farm Technical Appendix A8.1: Habitat Surveys
HEATHLAND WIND FARM TECHNICAL APPENDIX A8.1: HABITAT SURVEYS JANAURY 2021 Prepared By: Harding Ecology on behalf of: Arcus Consultancy Services 7th Floor 144 West George Street Glasgow G2 2HG T +44 (0)141 221 9997 l E [email protected] w www.arcusconsulting.co.uk Registered in England & Wales No. 5644976 Habitat Survey Report Heathland Wind Farm TABLE OF CONTENTS ABBREVIATIONS .................................................................................................................. 1 1 INTRODUCTION ........................................................................................................ 2 1.1 Background .................................................................................................... 2 1.2 Site Description .............................................................................................. 2 2 METHODS .................................................................................................................. 3 2.1 Desk Study...................................................................................................... 3 2.2 Field Survey .................................................................................................... 3 2.3 Survey Limitations .......................................................................................... 5 3 RESULTS .................................................................................................................... 6 3.1 Desk Study..................................................................................................... -

Volume 1, Chapter 2-7: Bryophyta
Glime, J. M. 2017. Bryophyta – Bryopsida. Chapt. 2-7. In: Glime, J. M. Bryophyte Ecology. Volume 1. Physiological Ecology. Ebook 2-7-1 sponsored by Michigan Technological University and the International Association of Bryologists. Last updated 10 January 2019 and available at <http://digitalcommons.mtu.edu/bryophyte-ecology/>. CHAPTER 2-7 BRYOPHYTA – BRYOPSIDA TABLE OF CONTENTS Bryopsida Definition........................................................................................................................................... 2-7-2 Chromosome Numbers........................................................................................................................................ 2-7-3 Spore Production and Protonemata ..................................................................................................................... 2-7-3 Gametophyte Buds.............................................................................................................................................. 2-7-4 Gametophores ..................................................................................................................................................... 2-7-4 Location of Sex Organs....................................................................................................................................... 2-7-6 Sperm Dispersal .................................................................................................................................................. 2-7-7 Release of Sperm from the Antheridium..................................................................................................... -

Phytochemical Studies of the Moss Species Plagiomnium Elatum and Plagiomnium Cuspidatum
journ. Hattori Bot. Lab. No. 67: 377- 382 (Dec. 1989) PHYTOCHEMICAL STUDIES OF THE MOSS SPECIES PLAGIOMNIUM ELATUM AND PLAGIOMNIUM CUSPIDATUM 1 S. ANHUT , T. SEEGER 1, J. B IEHL 1 , H. D . ZINSMEISTER 1 AND H . GEIGER 2 ABSTRACT. The flavonoid pattern of P/agiomnium cuspidatum and P. e/atum was evaluated. Fifteen dif ferent flavones, mainly flavone glycosides were isolated. The new natural compounds Elatin; 5-0H-Amento flavone; 2,3-Dihydro-5' -hydroxyamentoflavone, 2,3-Dihydro-5' -hydroxyrobustaflavone and 2,3-Dihydro- 5',3"'-dihydroxyamentoflavone were amongst them. The phytochemical relevance of these results is briefly discussed. INTRODUCTION The fi rst phytochemical studies on Mnium species was done by Kozlowski (1921). He detected Saponarin in Mnium cu:,pidatum, based on a chemical reaction with iodinepotassium iodide solution. Melchert & Alston (1965) reported on the occurrence of flavon C-glycosides in Mnium affine, McClure and Miller (1967) offlavonoids in Mnium cu.lpidatum. Koponen and Nilsson (1977) compared the flavonoid patterns of Plagiomnium elfipticum, P. medium, P. insigne, P. affine, P. tezukae, P. ciliare, P. ela/um, P. drummondii, P. japonicum, P. acutum and P. cuspidatum. Vandekerkhove (1977) isolated two f1avonoids from Mnium undulatum and characterized them as saponarin and an apigenin-6,8 di-C-glycoside. Further C- and C-O-glycosides from the same species were isolated and identified by Osterdahl (1979). Freitag et at. (1986) isolated two new isoorientin-O-diglycosides from P. affine. According to the "Generic revision of Mniaceae" (Koponen, 1968) all mentioned species were allocated to the genus Plagiomnium. The revised classification of the family Mniaceae resulted in four tribes with ten genera on the basis of morphological and karyological data. -

<I>Sphagnum</I> Peat Mosses
ORIGINAL ARTICLE doi:10.1111/evo.12547 Evolution of niche preference in Sphagnum peat mosses Matthew G. Johnson,1,2,3 Gustaf Granath,4,5,6 Teemu Tahvanainen, 7 Remy Pouliot,8 Hans K. Stenøien,9 Line Rochefort,8 Hakan˚ Rydin,4 and A. Jonathan Shaw1 1Department of Biology, Duke University, Durham, North Carolina 27708 2Current Address: Chicago Botanic Garden, 1000 Lake Cook Road Glencoe, Illinois 60022 3E-mail: [email protected] 4Department of Plant Ecology and Evolution, Evolutionary Biology Centre, Uppsala University, Norbyvagen¨ 18D, SE-752 36, Uppsala, Sweden 5School of Geography and Earth Sciences, McMaster University, Hamilton, Ontario, Canada 6Department of Aquatic Sciences and Assessment, Swedish University of Agricultural Sciences, SE-750 07, Uppsala, Sweden 7Department of Biology, University of Eastern Finland, P.O. Box 111, 80101, Joensuu, Finland 8Department of Plant Sciences and Northern Research Center (CEN), Laval University Quebec, Canada 9Department of Natural History, Norwegian University of Science and Technology University Museum, Trondheim, Norway Received March 26, 2014 Accepted September 23, 2014 Peat mosses (Sphagnum)areecosystemengineers—speciesinborealpeatlandssimultaneouslycreateandinhabitnarrowhabitat preferences along two microhabitat gradients: an ionic gradient and a hydrological hummock–hollow gradient. In this article, we demonstrate the connections between microhabitat preference and phylogeny in Sphagnum.Usingadatasetof39speciesof Sphagnum,withan18-locusDNAalignmentandanecologicaldatasetencompassingthreelargepublishedstudies,wetested -

Rhytidiadelphus Squarrosus
Rhytidiadelphus squarrosus Britain 1990–2013 2416 1950–1989 286 pre-1950 5 Ireland 1990–2013 590 1950–1989 180 pre-1950 4 robust moss with a wide ecological tolerance, occurring by heavy grazing and mowing, but is invariably absent from Aon all but the most acid soils in a variety of grassy reseeded and agriculturally improved fields. Altitudinal habitats, including sheep pastures, roadside and trackside range: 0–1170 m. verges, light woodland and scrub, dunes, streamsides, ditches and marshes. It also occurs among ericaceous shrubs Dioicous; capsules are rare in the lowlands but more and in flushed turf on heath and moorland. It is a very frequent in the north and west, mature in winter and early common species of lawns (where it attracts the attention and spring. Its ubiquity is remarkable, considering the rarity of often the disapproval of gardeners!), as well as churchyards, capsules and lack of gemmae. Presumably it is dispersed parkland and other places that are regularly mown and vegetatively by stem and leaf fragments. not heavily fertilised, and likewise it occurs in low-lying unimproved pastures. Regular associates include Lophocolea Plants from woodland often have a lax habit resembling bidentata, Brachythecium rutabulum, Calliergonella cuspidata, R. subpinnatus and the separation of the two species is Kindbergia praelonga and Pseudoscleropodium purum. It sometimes difficult. It is possible that there may be a few ascends to high altitudes in mountain grassland and Nardus recording errors in woodland habitats, but with little impact snowbeds, where associates include Hylocomium splendens, on the overall accuracy of the map. Pleurozium schreberi and Rhytidiadelphus loreus. -

Systematics and Ecology of the Moss Genus Scleropodium (Brachytheciaceae)
Systematics and ecology of the moss genus Scleropodium (Brachytheciaceae) By Benjamin Elias Carter A dissertation submitted in partial satisfaction of the requirements for the degree of Doctor of Philosophy in Integrative Biology in the Graduate Division of the University of California, Berkeley Committee in charge: Professor Brent D. Mishler, Chair Professor Bruce G. Baldwin Professor Chelsea D. Specht Spring 2012 Abstract Systematics and ecology of the moss genus Scleropodium (Brachytheciaceae) By Benjamin Elias Carter Doctor of Philosophy in Integrative Biology University of California, Berkeley Professor Brent D. Mishler, Chair Scleropodium is a genus of six species in the Brachytheciaceae. Although they are common in north temperate zones, they have not received monographic treatment in over a century. The aims of this study were to test species circumscriptions within the genus with molecular data, complete a thorough global taxonomic treatment of the genus, and to quantitatively investigate the ecological preferences of the species. A molecular phylogenetic study was conducted using 104 individuals spanning the range of morphological variation and the geographic extent of the genus. Maximum Parsimony and Bayesian phylogenetic analyses and a statistical parsimony network analysis of ITS and the chloroplast rps4, bsbA2 and trnG regions were performed. Although slight differences were found among analyses, there were six clear molecular groups. Five of these corresponded directly to the species Scleropodium californicum, S. cespitans, S. julaceum, S. obtusifolium and S. touretii. The sixth species, S. occidentale, is new to science and is described here. It is similar in ecology and morphology to S. obtusifolium, but has several diagnostic features in both molecular markers and morphological characters.