Wollemia Nobilis (Wollemi Pine)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Book of Abstracts.Pdf
1 List of presenters A A., Hudson 329 Anil Kumar, Nadesa 189 Panicker A., Kingman 329 Arnautova, Elena 150 Abeli, Thomas 168 Aronson, James 197, 326 Abu Taleb, Tariq 215 ARSLA N, Kadir 363 351Abunnasr, 288 Arvanitis, Pantelis 114 Yaser Agnello, Gaia 268 Aspetakis, Ioannis 114 Aguilar, Rudy 105 Astafieff, Katia 80, 207 Ait Babahmad, 351 Avancini, Ricardo 320 Rachid Al Issaey , 235 Awas, Tesfaye 354, 176 Ghudaina Albrecht , Matthew 326 Ay, Nurhan 78 Allan, Eric 222 Aydınkal, Rasim 31 Murat Allenstein, Pamela 38 Ayenew, Ashenafi 337 Amat De León 233 Azevedo, Carine 204 Arce, Elena An, Miao 286 B B., Von Arx 365 Bétrisey, Sébastien 113 Bang, Miin 160 Birkinshaw, Chris 326 Barblishvili, Tinatin 336 Bizard, Léa 168 Barham, Ellie 179 Bjureke, Kristina 186 Barker, Katharine 220 Blackmore, 325 Stephen Barreiro, Graciela 287 Blanchflower, Paul 94 Barreiro, Graciela 139 Boillat, Cyril 119, 279 Barteau, Benjamin 131 Bonnet, François 67 Bar-Yoseph, Adi 230 Boom, Brian 262, 141 Bauters, Kenneth 118 Boratyński, Adam 113 Bavcon, Jože 111, 110 Bouman, Roderick 15 Beck, Sarah 217 Bouteleau, Serge 287, 139 Beech, Emily 128 Bray, Laurent 350 Beech, Emily 135 Breman, Elinor 168, 170, 280 Bellefroid, Elke 166, 118, 165 Brockington, 342 Samuel Bellet Serrano, 233, 259 Brockington, 341 María Samuel Berg, Christian 168 Burkart, Michael 81 6th Global Botanic Gardens Congress, 26-30 June 2017, Geneva, Switzerland 2 C C., Sousa 329 Chen, Xiaoya 261 Cable, Stuart 312 Cheng, Hyo Cheng 160 Cabral-Oliveira, 204 Cho, YC 49 Joana Callicrate, Taylor 105 Choi, Go Eun 202 Calonje, Michael 105 Christe, Camille 113 Cao, Zhikun 270 Clark, John 105, 251 Carta, Angelino 170 Coddington, 220 Carta Jonathan Caruso, Emily 351 Cole, Chris 24 Casimiro, Pedro 244 Cook, Alexandra 212 Casino, Ana 276, 277, 318 Coombes, Allen 147 Castro, Sílvia 204 Corlett, Richard 86 Catoni, Rosangela 335 Corona Callejas , 274 Norma Edith Cavender, Nicole 84, 139 Correia, Filipe 204 Ceron Carpio , 274 Costa, João 244 Amparo B. -
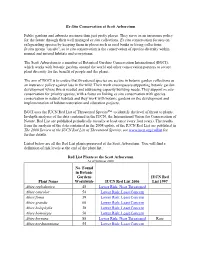
IUCN Red List of Threatened Species™ to Identify the Level of Threat to Plants
Ex-Situ Conservation at Scott Arboretum Public gardens and arboreta are more than just pretty places. They serve as an insurance policy for the future through their well managed ex situ collections. Ex situ conservation focuses on safeguarding species by keeping them in places such as seed banks or living collections. In situ means "on site", so in situ conservation is the conservation of species diversity within normal and natural habitats and ecosystems. The Scott Arboretum is a member of Botanical Gardens Conservation International (BGCI), which works with botanic gardens around the world and other conservation partners to secure plant diversity for the benefit of people and the planet. The aim of BGCI is to ensure that threatened species are secure in botanic garden collections as an insurance policy against loss in the wild. Their work encompasses supporting botanic garden development where this is needed and addressing capacity building needs. They support ex situ conservation for priority species, with a focus on linking ex situ conservation with species conservation in natural habitats and they work with botanic gardens on the development and implementation of habitat restoration and education projects. BGCI uses the IUCN Red List of Threatened Species™ to identify the level of threat to plants. In-depth analyses of the data contained in the IUCN, the International Union for Conservation of Nature, Red List are published periodically (usually at least once every four years). The results from the analysis of the data contained in the 2008 update of the IUCN Red List are published in The 2008 Review of the IUCN Red List of Threatened Species; see www.iucn.org/redlist for further details. -

Rare Or Threatened Vascular Plant Species of Wollemi National Park, Central Eastern New South Wales
Rare or threatened vascular plant species of Wollemi National Park, central eastern New South Wales. Stephen A.J. Bell Eastcoast Flora Survey PO Box 216 Kotara Fair, NSW 2289, AUSTRALIA Abstract: Wollemi National Park (c. 32o 20’– 33o 30’S, 150o– 151oE), approximately 100 km north-west of Sydney, conserves over 500 000 ha of the Triassic sandstone environments of the Central Coast and Tablelands of New South Wales, and occupies approximately 25% of the Sydney Basin biogeographical region. 94 taxa of conservation signiicance have been recorded and Wollemi is recognised as an important reservoir of rare and uncommon plant taxa, conserving more than 20% of all listed threatened species for the Central Coast, Central Tablelands and Central Western Slopes botanical divisions. For a land area occupying only 0.05% of these divisions, Wollemi is of paramount importance in regional conservation. Surveys within Wollemi National Park over the last decade have recorded several new populations of signiicant vascular plant species, including some sizeable range extensions. This paper summarises the current status of all rare or threatened taxa, describes habitat and associated species for many of these and proposes IUCN (2001) codes for all, as well as suggesting revisions to current conservation risk codes for some species. For Wollemi National Park 37 species are currently listed as Endangered (15 species) or Vulnerable (22 species) under the New South Wales Threatened Species Conservation Act 1995. An additional 50 species are currently listed as nationally rare under the Briggs and Leigh (1996) classiication, or have been suggested as such by various workers. Seven species are awaiting further taxonomic investigation, including Eucalyptus sp. -

Kew Science Publications for the Academic Year 2017–18
KEW SCIENCE PUBLICATIONS FOR THE ACADEMIC YEAR 2017–18 FOR THE ACADEMIC Kew Science Publications kew.org For the academic year 2017–18 ¥ Z i 9E ' ' . -,i,c-"'.'f'l] Foreword Kew’s mission is to be a global resource in We present these publications under the four plant and fungal knowledge. Kew currently has key questions set out in Kew’s Science Strategy over 300 scientists undertaking collection- 2015–2020: based research and collaborating with more than 400 organisations in over 100 countries What plants and fungi occur to deliver this mission. The knowledge obtained 1 on Earth and how is this from this research is disseminated in a number diversity distributed? p2 of different ways from annual reports (e.g. stateoftheworldsplants.org) and web-based What drivers and processes portals (e.g. plantsoftheworldonline.org) to 2 underpin global plant and academic papers. fungal diversity? p32 In the academic year 2017-2018, Kew scientists, in collaboration with numerous What plant and fungal diversity is national and international research partners, 3 under threat and what needs to be published 358 papers in international peer conserved to provide resilience reviewed journals and books. Here we bring to global change? p54 together the abstracts of some of these papers. Due to space constraints we have Which plants and fungi contribute to included only those which are led by a Kew 4 important ecosystem services, scientist; a full list of publications, however, can sustainable livelihoods and natural be found at kew.org/publications capital and how do we manage them? p72 * Indicates Kew staff or research associate authors. -

Pdf Environment and Conservation, Hurstville
References and further reading Scientific Committee (2015). Hibbertia sp. Turramurra Final Determination NSW Threatened Species Scientific Committee. Keith, D.A. (2004). Ocean shores to desert dunes: the native Available from www.environment.nsw.gov.au/resources/ vegetation of New South Wales and the A.C.T. Department of threatenedspecies/determinations/FDHibbTurraCR.pdf Environment and Conservation, Hurstville. Threatened Species Scientific Committee (2016). Conservation OEH (2013). The Native Vegetation of the Sydney Metropolitan Advice Hibbertia spanantha Julian’s hibbertia. Department of the Area (Version 2.0). Office of Environment and Heritage, Environment and Energy, Canberrra. Department of Premier and Cabinet, Sydney. Toelken, H.R. and Robinson, A.F. (2015). Notes on Hibbertia OEH (2017). Julian’s Hibbertia-profile. Office of Environment (Dilleniaceae) 11. Hibbertia spanantha, a new species from the and Heritage NSW Government, Sydney. Accessed 6th central coast of New South Wales. Journal of the Adelaide Botanic February 2018 Available from www.environment.nsw.gov.au/ Gardens 29: 11–14 threatenedSpeciesApp/profile.aspx?id=20279 Threatened plant translocation case study: Wollemia nobilis (Wollemi Pine), Araucariaceae HEIDI ZIMMER1*, PATRICK BAKER2, CATHERINE OFFORD3, JESSICA RIGG4, GREG BOURKE5 AND TONY AULD1 1 NSW Office of Environment and Heritage 2 University of Melbourne 3 The Australian Botanic Garden Mount Annan 4 NSW Department of Primary Industries 5 The Blue Mountains Botanic Garden Mount Tomah *Corresponding author: [email protected] The species There are around 200 seedlings and juvenile Wollemi Pines in the wild. It is likely that the creation of canopy The Wollemi Pine is a Critically Endangered conifer that is gaps would increase Wollemi Pine recruitment, as many endemic to a single catchment in Wollemi National Park, rainforest trees. -

Gymnosperms on the EDGE Félix Forest1, Justin Moat 1,2, Elisabeth Baloch1, Neil A
www.nature.com/scientificreports OPEN Gymnosperms on the EDGE Félix Forest1, Justin Moat 1,2, Elisabeth Baloch1, Neil A. Brummitt3, Steve P. Bachman 1,2, Stef Ickert-Bond 4, Peter M. Hollingsworth5, Aaron Liston6, Damon P. Little7, Sarah Mathews8,9, Hardeep Rai10, Catarina Rydin11, Dennis W. Stevenson7, Philip Thomas5 & Sven Buerki3,12 Driven by limited resources and a sense of urgency, the prioritization of species for conservation has Received: 12 May 2017 been a persistent concern in conservation science. Gymnosperms (comprising ginkgo, conifers, cycads, and gnetophytes) are one of the most threatened groups of living organisms, with 40% of the species Accepted: 28 March 2018 at high risk of extinction, about twice as many as the most recent estimates for all plants (i.e. 21.4%). Published: xx xx xxxx This high proportion of species facing extinction highlights the urgent action required to secure their future through an objective prioritization approach. The Evolutionary Distinct and Globally Endangered (EDGE) method rapidly ranks species based on their evolutionary distinctiveness and the extinction risks they face. EDGE is applied to gymnosperms using a phylogenetic tree comprising DNA sequence data for 85% of gymnosperm species (923 out of 1090 species), to which the 167 missing species were added, and IUCN Red List assessments available for 92% of species. The efect of diferent extinction probability transformations and the handling of IUCN data defcient species on the resulting rankings is investigated. Although top entries in our ranking comprise species that were expected to score well (e.g. Wollemia nobilis, Ginkgo biloba), many were unexpected (e.g. -

Curriculum Vitae
CURRICULUM VITAE ORCID ID: 0000-0003-0186-6546 Gar W. Rothwell Edwin and Ruth Kennedy Distinguished Professor Emeritus Department of Environmental and Plant Biology Porter Hall 401E T: 740 593 1129 Ohio University F: 740 593 1130 Athens, OH 45701 E: [email protected] also Courtesy Professor Department of Botany and PlantPathology Oregon State University T: 541 737- 5252 Corvallis, OR 97331 E: [email protected] Education Ph.D.,1973 University of Alberta (Botany) M.S., 1969 University of Illinois, Chicago (Biology) B.A., 1966 Central Washington University (Biology) Academic Awards and Honors 2018 International Organisation of Palaeobotany lifetime Honorary Membership 2014 Fellow of the Paleontological Society 2009 Distinguished Fellow of the Botanical Society of America 2004 Ohio University Distinguished Professor 2002 Michael A. Cichan Award, Botanical Society of America 1999-2004 Ohio University Presidential Research Scholar in Biomedical and Life Sciences 1993 Edgar T. Wherry Award, Botanical Society of America 1991-1992 Outstanding Graduate Faculty Award, Ohio University 1982-1983 Chairman, Paleobotanical Section, Botanical Society of America 1972-1973 University of Alberta Dissertation Fellow 1971 Paleobotanical (Isabel Cookson) Award, Botanical Society of America Positions Held 2011-present Courtesy Professor of Botany and Plant Pathology, Oregon State University 2008-2009 Visiting Senior Researcher, University of Alberta 2004-present Edwin and Ruth Kennedy Distinguished Professor of Environmental and Plant Biology, Ohio -

Wollemi Pine (Wollemia Nobilis) and Its Introduction to Cultivation in Great Britain and Ireland©
432 Combined Proceedings International Plant Propagators’ Society, Volume 58, 2008 Wollemi Pine (Wollemia nobilis) and Its Introduction to Cultivation in Great Britain and Ireland© Mark Taylor Kernock Park Plants, Pillaton, Saltash, Cornwall PL12 6RY Email: [email protected] INTRODUCTION The Wollemi pine (Wollemia nobilis) was discovered within the Wollemi National Park, a virtually untouched wilderness area of 361,000 ha in the Blue Mountains, 200 km north west of Sydney Australia. It was found on 10 Sept. 1994, when a park ranger was exploring the deep canyons in the park. Wollemi is an Australian Ab- original word which means “stop, and look around you.” The ranger, David Noble, knew most of the tree species in the Park and he must have followed this Aboriginal saying when he stumbled across a grove of enormous trees unlike any that he had seen before. When I spoke to him on his visit to the U.K. in April 2006, he said it was a very strange sensation being in that canyon and even then, it evoked feelings and thoughts of the fairly recent film of that time, Jurassic Park. Noble collected a section of foliage to show his colleagues at the New South Wales National Parks and Wildlife Service. It was soon established that the tree belonged to the Araucariaceae, the same family as the monkey puzzle, Norfolk Island pine, and less well known trees such as the hoop, bunya, and kauri pines. Further inves- tigation, including help from the Royal Botanic Gardens, Sydney, revealed that this was, in fact, a completely new genus of tree. -

The Mediterranean Palynological Societies Symposium 2019
The Mediterranean Palynological Societies Symposium 2019. Abstract book. Stéphanie Desprat, Anne-Laure Daniau, Maria Fernanda Sánchez Goñi To cite this version: Stéphanie Desprat, Anne-Laure Daniau, Maria Fernanda Sánchez Goñi. The Mediterranean Palyno- logical Societies Symposium 2019. Abstract book.. MedPalyno 2019, Jul 2019, Bordeaux, France. Université de Bordeaux, pp.142, 2019, 978-2-9562881-3-8. hal-02274992 HAL Id: hal-02274992 https://hal.archives-ouvertes.fr/hal-02274992 Submitted on 30 Aug 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. ABSTRACT BOOK The Mediterranean Palynological Societies Symposium 2019 The joint symposium of the APLF, APLE and GPP-SBI Bordeaux, July 9-10-11, 2019 Title: The Mediterranean Palynological Societies Symposium 2019. Abstract book. Editors: St´ephanie Desprat, Anne-Laure Daniau and Mar´ıa Fernanda S´anchez Go˜ni Publisher: Université de Bordeaux IBSN: 978-2-9562881-3-8 E-book available on https://hal.archives-ouvertes.fr/ ORGANIZING COMMITTEE Local committee from the EPOC research unit (UMR 5805: CNRS, Universite´ de Bordeaux, EPHE) Charlotte Clement´ Anne-Laure Daniau Stephanie´ Desprat Ludovic Devaux Tiffanie Fourcade Marion Genet Muriel Georget Laurent Londeix Maria F. Sanchez Goni˜ Coralie Zorzi Enlarged committee - Presidents of the APLF, GPPSBI and APLE Vincent Lebreton, HNHP, UMR 7194 CNRS-Mus´eumNational d’Histoire Naturelle (MNHN)-UPVD (France) Anna Maria Mercuri, Universit`adegli Studi di Modena e Reggio Emilia, Department of Life Sciences (Italy) Pilar S. -

The Seed Cone Eathiestrobus Gen. Nov.: Fossil Evidence for a Jurassic
American Journal of Botany 99(4): 708–720. 2012. T HE SEED CONE E ATHIESTROBUS GEN. NOV.: 1 F OSSIL EVIDENCE FOR A JURASSIC ORIGIN OF PINACEAE G AR W . R OTHWELL 2,3,6 , G ENE M APES 2 , R UTH A . S TOCKEY 3,4 AND J ASON H ILTON 5 2 Department of Environmental and Plant Biology, Ohio University, Athens, Ohio 45701 USA; 3 Department of Botany and Plant Pathology, Oregon State University, Corvallis, Oregon 97331 USA; 4 Department of Biological Sciences, University of Alberta, Edmonton, AB, T6G 2E9, Canada; and 5 School of Geography, Earth and Environmental Sciences, University of Birmingham, Edgbaston, Birmingham, B15 2TT, UK • Premise of the study: Pinaceae and nonpinoid species are sister groups within the conifer clade as inferred from molecular systematic comparisons of living species and therefore should have comparable geological ages. However, the fossil record for the nonpinoid lineage of extant conifer families is Triassic, nearly 100 million years older than the oldest widely accepted Lower Cretaceous record for Pinaceae. An anatomically preserved fossil conifer seed cone described here extends the strati- graphic range of Pinaceae nearly 30 million years, thus reducing the apparent discrepancy between evidence from the fossil record and inferences from systematic studies of living species. • Methods: Material was prepared as serial thin sections by the cellulose acetate peel technique, mounted on microscope slides, and viewed and photographed using transmitted light. • Key results: A large cylindrical cone consisting of bract-scale complexes that diverge from the cone axis in a helical phyllotaxis has bracts and scales that separate from each other in the midregion and are of equal length and of nearly equal width. -

Cenozoic Araucariaceae in the Northern Hemisphere
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Wulfenia Jahr/Year: 2015 Band/Volume: 22 Autor(en)/Author(s): Oskolski Alexei A. Artikel/Article: Cenozoic Araucariaceae in the Northern hemisphere: decline and recovery 261-264 © Landesmuseum für Kärnten; download www.landesmuseum.ktn.gv.at/wulfenia; www.zobodat.at Wulfenia 22 (2015): 261–264 Mitteilungen des Kärntner Botanikzentrums Klagenfurt Cenozoic Araucariaceae in the Northern hemisphere: decline and recovery Alexei A. Oskolski Summary: Cenozoic extinct taxa of Araucariaceae from Europe, Asia and Northern America have been surveyed. The macrofossil and palynological records of Araucariaceae in the Northern hemisphere end in the Eocene. There are no convincing data on the occurrence of this family in the early Oligocene of Eurasia and North America. Two araucarioid fossil woods that have been reported (Prakash & Du 1995; Feng et al. 2015) from the late Oligocene to middle Miocene of China (Araucarioxylon shandongense Prakash & Du from Shandong province and Agathoxylon sp. from Hainan island) are the most recent fossil records of Araucariaceae in the Northern hemisphere. These woods may be considered as evidence of the dispersal of Araucariaceae from Australia to East Asia that gave rise to radiation of the genus Agathis. Keywords: Araucariaceae, Cenozoic, Paleogene, Northern hemisphere, Oligocene, Miocene, Araucarioxylon shandongense, Agathoxylon, Agathis The conifer family Araucariaceae comprising three recent genera Araucaria, Agathis and Wollemia is distributed now in South America, Malesia, Australia and Western Pacific. This family appeared in the early Triassic and widely expanded and diversified during the Mesozoic. In contrast to the present range, the Araucariaceae were widespread in both hemispheres throughout Jurassic and Cretaceous (Stockey 1982; Kershaw & Wagstaff 2001; Leslie et al. -

Araucaria Bidwillii Araucariaceae G
Araucaria bidwillii G. Don Araucariaceae LOCAL NAMES English (bunya pine,bunya bunya pine,bunya bunya) BOTANIC DESCRIPTION Araucaria bidwillii is a fast-growing tree 30-50 m tall, with a diameter of 1.5 m, and a straight, undivided trunk often free of branches for two-thirds of the tree height and showing little taper in this part of the bole. The crown is normally symmetrical and dome shaped (parabolic), tending to change from a pointed to a flattened apex with age. The drooping branches are themselves unbranched, with the leaves clustered at the ends. As the Foliage (Dennis Haugen, lower branches die, dormant buds become active at the base of each www.forestryimages.org) branch and a secondary, dome-shaped crown may develop below the primary one. Bark is persistent over the trunk and branches, with thin scales up to 2.5 x 7.5 cm. The outer surface of the bark is rough, bumpy, dark brown to black, covered with prominent leaf scars, and the cut blaze is red, grading to orange. Juvenile leaves most commonly seen in double rows along the branchlets spirally arranged, venation not visible, glossy green, triangular in section, stiff; adult leaf shiny, tough, sharp-tipped, glossy green, discolorous, 2-6 cm long, 0.5-1 cm wide, spirally arranged but become two-ranked through twisting of the leaf bases. They are stalkless or very shortly petiolate, tree (Dennis Haugen, lanceolate. www.forestryimages.org) Male and female strobili are usually borne on the same tree. Males up to 20 cm long, produced at the ends of short, lateral branches and made up of numerous spirally arranged scales, each with a diamond-shaped, expanded summit covering about 12 pollen cells.