Full Text PDF (221K)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Blood-Aqueous Barrier Changes After the Use of Prostaglandin Analogues in Patients with Pseudophakia and Aphakia a 6-Month Randomized Trial
CLINICAL SCIENCES Blood-Aqueous Barrier Changes After the Use of Prostaglandin Analogues in Patients With Pseudophakia and Aphakia A 6-Month Randomized Trial Enyr S. Arcieri, MD; Alessandro Santana, MD; Fabiano N. Rocha, MD; Gustavo L. Guapo, MD; Vital P. Costa, MD Objectives: To investigate the effects of prostaglandin throughout follow-up (P Ͻ .02). Four latanoprost- analogues on the blood-aqueous barrier and to evaluate treated eyes, 1 bimatoprost-treated eye, and 1 travoprost- the occurrence of cystoid macular edema in aphakic or treated eye developed cystoid macular edema; all cases pseudophakic patients with glaucoma. resolved after discontinuation of the prostaglandin ana- logue and treatment with topical diclofenac sodium. Mean Methods: In this randomized, masked-observer, 6-month intraocular pressure reductions after 6 months were higher clinical trial, patients with primary open-angle, pseudo- for the latanoprost (26%), bimatoprost (28%), and tra- phakic, or aphakic glaucoma were treated once daily with voprost (29%) groups than for the control (3%) and uno- bimatoprost (n=16), latanoprost (n=15), or travoprost prostone (14%) groups (PϽ.05). Bimatoprost induced (n=17) or twice daily with unoprostone (n=16) or lu- significantly higher hyperemia scores than latanoprost, bricant drops (control group) (n=16). Blood-aqueous bar- unoprostone, and placebo (PϽ.01). rier status, which was assessed using a laser flare meter; intraocular pressure; the occurrence of angiographic cys- toid macular edema; and conjunctival hyperemia were Conclusion: Bimatoprost, latanoprost, and travoprost use evaluated. may lead to disruption of the blood-aqueous barrier in patients with pseudophakia and aphakia. Results: Mean flare values were significantly higher in the bimatoprost, latanoprost, and travoprost groups Arch Ophthalmol. -

New York State Medicaid Drug Utilization Board Meeting Agenda
New York State Medicaid Drug Utilization Board Meeting Agenda The Drug Utilization (DUR) Board will meet April 24, 2014, from 9:00 a.m. to 4:30 p.m., Meeting Room 6, Concourse, Empire State Plaza, Albany, New York Agenda Items Preferred Drug Program (PDP) The DUR Board will review therapeutic classes, described and listed below, as they pertain to the PDP. The therapeutic classes to be reviewed contain new relevant clinical and/or financial information. Therapeutic classes not included on this agenda may be reviewed at a later date. The Board will review new clinical and financial information as required, to recommend preferred and non-preferred drugs. ^ The Board will only consider clinical information which is new since the previous review of the therapeutic class and then consider financial information. New clinical information may include a new drug or drug product information, new indications, new safety information or new published clinical trials (comparative evidence is preferred, or placebo controlled when no head-to-head trials are available). Information in abstract form alone, posters, or unpublished data are poor quality evidence for the purpose of re-review and submission is discouraged. Those wishing to submit new clinical information must do so in an electronic format by April 9, 2014 or the Board may not have ample time to review the information. ^ The current preferred and non-preferred status of drugs subject to the Preferred Drug List (PDL) may be viewed at https://newyork.fhsc.com/downloads/providers/NYRx_PDP_PDL.pdf -

Effect of Latanoprost Or 8-Iso Prostaglandin E2 Alone and in Combination on Intraocular Pressure in Glaucomatous Monkey Eyes
LABORATORY SCIENCES Effect of Latanoprost or 8-iso Prostaglandin E2 Alone and in Combination on Intraocular Pressure in Glaucomatous Monkey Eyes Rong-Fang Wang, MD; Steven M. Podos, MD; Janet B. Serle, MD; Thomas W. Mittag, PhD; F. Ventosa, MD; Bernard Becker, MD Objective: To evaluate the possible additivity of the ef- duction of IOP of 4.0 ± 0.6 mm Hg was produced when fects of latanoprost and 8-iso prostaglandin E2 (8-iso PGE2) 8-iso PGE2 was added to latanoprost and of 3.0 ± 0.7 on intraocular pressure (IOP) in monkey eyes with laser- mm Hg was produced when latanoprost was added to 8-iso induced glaucoma. PGE2 on day 13 before the morning dosing. Combina- tion therapy with both agents caused maximum IOP re- Methods: The IOP was measured hourly for 6 hours be- ductions from baseline of 11.3 ± 3.0 mm Hg (33%) (P,.05) ginning at 9:30 AM on day 1 (baseline day), days 6 and 7 (latanoprost with 8-iso PGE2 added) and of 9.8 ± 1.3 , (single-agent therapy), and days 13 and 14 (combination mm Hg (31%) (P .01) (8-iso PGE2 with latanoprost added) therapy with both agents). Following 1 day of baseline mea- on day 14. surement, 4 monkeys with unilateral glaucoma received monotherapy twice daily with either 1 drop of 0.005% la- Conclusion: Latanoprost and 8-iso PGE2 have an addi- tanoprost, or 0.1% 8-iso PGE2, 25 µL, at 9:30 AM and 3:30 tive effect on IOP in glaucomatous monkey eyes. -

Unoprostone Isopropyl (Rescula) Reference Number: HIM.PA.11 Effective Date: 09.04.18 Last Review Date: 11.19 Revision Log Line of Business: HIM
Clinical Policy: Unoprostone Isopropyl (Rescula) Reference Number: HIM.PA.11 Effective Date: 09.04.18 Last Review Date: 11.19 Revision Log Line of Business: HIM See Important Reminder at the end of this policy for important regulatory and legal information. Description Unoprostone isopropyl ophthalmic solution, 0.15% (Rescula®) is a synthetic analog (docosanoid) of prostaglandin F2-alpha. FDA Approved Indication(s) Rescula is indicated for the reduction of elevated intraocular pressure in patients with open-angle glaucoma or ocular hypertension. Policy/Criteria Provider must submit documentation (such as office chart notes, lab results or other clinical information) supporting that member has met all approval criteria. It is the policy of health plans affiliated with Centene Corporation® that Rescula is medically necessary when the following criteria are met: I. Initial Approval Criteria A. Ocular Hypertension or Open-Angle Glaucoma (must meet all): 1. Diagnosis of open-angle glaucoma or ocular hypertension; 2. Failure of prostaglandin analogues (e.g., latanoprost, travoprost, bimatoprost) in combination with beta blockers (e.g., timolol), unless contraindicated or clinically significant adverse effects are experienced; 3. Age ≥ 18 years; 4. Dose does not exceed 1 bottle per 35 days. Approval duration: 12 months B. Other diagnoses/indications 1. Refer to the off-label use policy for the relevant line of business if diagnosis is NOT specifically listed under section III (Diagnoses/Indications for which coverage is NOT authorized): HIM.PHAR.21 for health insurance marketplace. II. Continued Therapy A. Ocular Hypertension or Open-Angle Glaucoma (must meet all): 1. Currently receiving medication via Centene benefit or member has previously met initial approval criteria; 2. -
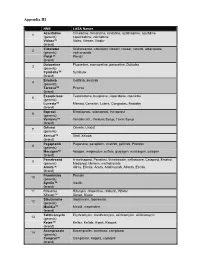
Appendix III
Appendix III NME LASA Names Azacitidine Cimetidine, famotidine, ranitidine, azathioprine, azulfidine, 1 (generic) capecitabine, zalcitabine Vidaza™ Videx, Vitrase, Viadur (brand) Cilostazol Sulfisoxazole, colestipol, clozaril, cozaar, inositol, aldactazide, 2 (generic) voriconazole Pletal™ Plendil (brand) Duloxetine Fluoxetine, atomoxetine, paroxetine, Dulcolax 3 (generic) Cymbalta™ Symbyax (brand) Erlotinib Gefitinib, imatinib 4 (generic) Tarceva™ Pexeva (brand) Eszopiclone Testolactone, buspirone, risperidone, ropinirole 5 (generic) Lunesta™ Menest, Cenestin, Lutera, Congestac, Nestabs (brand) Iloprost Bimatoprost, latanoprost, travoprost 6 (generic) Ventavis™ Ventolin inh., Ventuss Syrup, Tavist Syrup (brand) Orlistat Orlenta, Uristat 7 (generic) Xenical™ Xiral, Xeloda (brand) Pegaptanib Paganone, paraplatin, imatinib, gefitinib, Protonix 8 (generic) Macugen™ Adagen, magnesium sulfate, glucagen, mustargen, salagen (brand) Pemetrexed A-methapred, Penetrex, trimetrexate, ceftriaxone, Cetapred, Emetrol, 9 (generic) Medipred, Merrem, methotrexate Alimta™ Alinia, Elimite, Aceta, Adalimunab, Alfenta, Esclim (brand) Pramlintide Prandin 10 (generic) Symlin™ Insulin (brand) 11 Rifaximin Rifampin, rifapentine, rifabutin, Rifater Xifaxan™ Xanax, Biaxin Sibutramine Imipramine, topiramate 12 (generic) Meridia™ Mexitil, meperidine (brand) Telithromycin Erythromycin, clarithromycin, azithromycin, dirithromycin 13 (generic) Ketek™ Keflex, Keftab, K-pek, Kaopek (brand) Acamprosate Bacampicillin, acarbose, camptosar 14 (generic) Campral™ Camptosar, Keppra, -

Adverse Periocular Reactions to Five Types of Prostaglandin Analogs
Eye (2012) 26, 1465–1472 & 2012 Macmillan Publishers Limited All rights reserved 0950-222X/12 www.nature.com/eye 1 1 1 1 Adverse periocular K Inoue , M Shiokawa , R Higa , M Sugahara , CLINICAL STUDY T Soga1, M Wakakura1 and G Tomita2 reactions to five types of prostaglandin analogs Abstract Purpose We investigated the appearance explained to patients before PG frequency of eyelid pigmentation and administration. eyelash bristles after the use of five types of Eye (2012) 26, 1465–1472; doi:10.1038/eye.2012.195; prostaglandin (PG) analogs. published online 5 October 2012 Methods This study included 250 eyes from 250 patients diagnosed with primary open- Keywords: prostaglandin analogs; adverse angle glaucoma or ocular hypertension who reactions; eyelid pigmentation; eyelash bristles; were treated with either latanoprost, patient’s subjective evaluation; physician’s travoprost, tafluprost, bimatoprost, or subjective evaluation isopropyl unoprostone for 43 months in only one eye. Photographs of both eyes were obtained, and the images were assessed by Introduction three ophthalmologists who were masked to treatment type. The existence of eyelid Prostaglandin (PG) analogs are the primary pigmentation and eyelash bristles was treatment for glaucoma because of their judged, and images of the left and right eyes powerful intraocular pressure (IOP) decreasing were compared. Subjective symptoms effect, few systematic adverse reactions, and regarding the existence of eyelid convenience of once a day administration (other pigmentation and eyelash bristles were than isopropyl unoprostone (unoprostone)).1 Five types of PG analogs (latanoprost, investigated through a questionnaire. 1Inouye Eye Hospital, Tokyo, Results There was no significant difference travoprost, tafluprost, bimatoprost, and Japan between the five types of medications with unoprostone) are currently available in Japan. -

Unoprostone As Adjunctive Therapy to Timolol: a Double Masked
592 CLINICAL SCIENCE Br J Ophthalmol: first published as 10.1136/bjo.87.5.592 on 1 May 2003. Downloaded from Unoprostone as adjunctive therapy to timolol: a double masked randomised study versus brimonidine and dorzolamide A Hommer, for The Unoprostone Adjunctive Therapy Study Group,* B Kapik, N Shams ............................................................................................................................. Br J Ophthalmol 2003;87:592–598 *Members listed at the end Aims: To compare the safety and efficacy of unoprostone, brimonidine, and dorzolamide as adjunc- of paper. tive therapy to timolol in patients with primary open angle glaucoma or ocular hypertension. Methods: This was a randomised, double masked, parallel group, multicentre (14) study. After using timolol maleate 0.5% monotherapy twice a day for 2 weeks, patients (n = 146) with an early morning intraocular pressure (IOP) between 22 and 28 mm Hg, inclusively, received unoprostone isopropyl 0.15% (n = 50), brimonidine tartrate 0.2% (n = 48), or dorzolamide hydrochloride 2.0% (n = 48) twice daily as adjunctive therapy to timolol maleate 0.5% for another 12 weeks. Safety was based on comprehensive ophthalmic examinations, adverse events, and vital signs. Efficacy was based on mean change from baseline in the 8 hour diurnal IOP at week 12. Baseline was defined as values obtained See end of article for after 2 weeks of timolol monotherapy. authors’ affiliations Results: Each drug was safe and well tolerated. Burning/stinging was the most common treatment ....................... emergent adverse event. No clinically relevant changes from baseline were observed for any ophthal- Correspondence to: mic examination or vital signs. At week 12, each adjunctive therapy produced statistically significant Naveed Shams, MD, PhD, (p<0.001) reductions from timolol treated baseline in the mean 8 hour diurnal IOP (−2.7 mm Hg, uno- Novartis Ophthalmics Inc, prostone; −2.8 mm Hg, brimonidine; −3.1 mm Hg, dorzolamide). -

Additive Effect of Unoprostone and Latanoprost in Patients with Elevated Intraocular Pressure
75 CLINICAL SCIENCE Br J Ophthalmol: first published as 10.1136/bjo.86.1.75 on 1 January 2002. Downloaded from Additive effect of unoprostone and latanoprost in patients with elevated intraocular pressure Tin Aung, Paul T K Chew, Francis T S Oen, Yiong-Huak Chan, Lennard H Thean, Leonard Yip, Boon-Ang Lim, Steve K L Seah ............................................................................................................................. Br J Ophthalmol 2002;86:75–79 Aims: To assess the additive effect of unoprostone and latanoprost in patients with primary open angle glaucoma (POAG) or ocular hypertension (OHT) Methods: 32 patients with POAG or OHT were randomised to receive either latanoprost once daily or unoprostone twice daily for 4 weeks. After 4 weeks, all patients received both latanoprost and unoprostone for another 4 weeks. The IOP was measured at 9 am and 5 pm on the baseline, day 28, and day 56 visits, and at 9 am on day 14 and day 42 visits. The medications were given to the patients in an open label fashion. The observer was masked to the treatment given. The mean of the measure- ments was calculated. Safety parameters were also recorded. The additive effect of the medications was assessed by the reduction in intraocular pressure (IOP) when both medications were used, compared with when one medication was used. Results: 28 patients completed both treatment periods and had IOP data available for evaluation. See end of article for After 1 month of treatment, latanoprost significantly reduced IOP (mean by 6.1 (SEM 0.8) mm Hg authors’ affiliations (p<0.001) and unoprostone by 4.9 (1.0) mm Hg (p<0.001) from the baseline of 24.4 (0.6) mm Hg ...................... -

Effects of Topical Antiglaucoma Medications on Corneal Epithelium As Evaluated by Gene Expression Patterns Yuka Okada, MD, Phd
JOBNAME: corn 26#Suppl. 1 2007 PAGE: 1 OUTPUT: Thursday July 26 14:59:59 2007 tsp/corn/146793/ICO200709 ARTICLE Effects of Topical Antiglaucoma Medications on Corneal Epithelium as Evaluated by Gene Expression Patterns Yuka Okada, MD, PhD Key Words: cyclooxygenase-2, antiglaucoma medication, corneal Purpose: To examine the expression pattern of the stress-related epithelium, transcription factor genes c-fos and c-jun, which encode the 2 major components of activator protein (AP)-1, and cyclooxygenase (COX)-2 in rat corneal (Cornea 2007;26(Suppl. 1):S46–S54) epithelium treated with topical antiglaucoma medications and benzalkonium chloride (BAK) preservative. Methods: Eighty-eight male Wistar rats were used. We instilled antiglaucoma eye drops (0.5% Timoptol, 0.005% Xalatan, or 0.12% opical b-blocker (timolol, 0.5% Timoptol) and prosta- Rescula), their chemical constituents (active ingredients), or BAK Tglandin (PG)F2a analogs (latanoprost, Xalatan; isopropyl preservative (0.005%, 0.01%, or 0.02%) in 1 eye of each rat. Fellow unoprostone, Rescula) are widely used in the management of eyes served as controls. The eyes were enucleated after various glaucoma and ocular hypertension. Because antiglaucoma intervals. In situ hybridization and immunohistochemistry were used drugs are used for long time periods, chronic side effects are to detect expression of c-fos, c-jun, and COX-2. a major concern. Among these side effects, ocular surface disorders attributable to the drug itself or to drug preser- Results: Expression of c-fos, c-jun, and COX-2 was minimally vatives are relatively common.1–4 Benzalkonium chloride observed in uninjured rat corneal epithelium. -

Download Product Insert (PDF)
PRODUCT INFORMATION Tafluprost ethyl ester Item No. 11612 CAS Registry No.: 209860-89-9 Formal Name: 15,15-difluoro-9α,11α-dihydroxy-16- phenoxy-17,18,19,20-tetranor-prosta- OH 5Z,13E-dien-1-oic acid, ethyl ester Synonym: AFP 175 COOCH2CH3 MF: C24H32F2O5 FW: 438.5 HO O Purity: ≥98% F F UV/Vis.: λmax: 217 nm Supplied as: 10% wt/wt in ethanol Storage: -20°C Stability: ≥2 years Information represents the product specifications. Batch specific analytical results are provided on each certificate of analysis. Laboratory Procedures Tafluprost ethyl ester is supplied as 10% wt/wt in ethanol. To change the solvent, simply evaporate the ethanol under a gentle stream of nitrogen and immediately add the solvent of choice. Solvents such as DMSO and dimethyl formamide purged with an inert gas can be used. The solubility of tafluprost ethyl ester in these solvents is approximately 30 mg/ml. Description Analogs of prostaglandin F2α (PGF2α), which act primarily at the FP receptor, have been approved for 1-4 the treatment of glaucoma. Tafluprost (free acid) (Item No. 10005439) is a PGF2α analog which acts as 5 a potent FP receptor agonist (Ki = 0.4 nM). Tafluprost ethyl ester is a form of tafluprost which may have increased lipid solubility compared to the free acid. In addition, the ethyl ester form may demonstrate improved uptake into tissues and thus lower the effective concentration. References 1. Parrish, R.K., Palmberg, P., and Sheu, W.-P. A comparison of latanoprost, bimatoprost, and travoprost in patients with elevated intraocular pressure: A 12-week, randomized, masked-evaluator multicenter study. -

Effect of Non-Steroidal Anti-Inflammatory Ophthalmic Solution on Intraocular Pressure Reduction by Latanoprost K Kashiwagi, S Tsukahara
297 CLINICAL SCIENCE Br J Ophthalmol: first published as 10.1136/bjo.87.3.297 on 1 March 2003. Downloaded from Effect of non-steroidal anti-inflammatory ophthalmic solution on intraocular pressure reduction by latanoprost K Kashiwagi, S Tsukahara ............................................................................................................................. Br J Ophthalmol 2003;87:297–301 Aim: To investigate the effects of a non-steroidal anti-inflammatory drug (NSAID) ophthalmic solution on latanoprost induced intraocular pressure (IOP) reduction using normal volunteers. Methods: This study was conducted as a prospective and observer masked clinical trial. 13 normal volunteers were enrolled. After measurement of basal IOP and ophthalmic examination, latanoprost ophthalmic solution was initially administered to both eyes once daily. Four weeks later, an NSAID ophthalmic solution, sodium 2-amino-3-(4-bromobenzoyl) phenylacetate sesquihydrate (refer to bromfenac sodium hydrate), was co-administered to one randomly selected eye (NSAID group) twice daily for 2 weeks. The other eye was employed as a control (non-NSAID group). After withdrawal of the NSAID ophthalmic solution, latanoprost ophthalmic solution was continuously administered for another 2 weeks and was then withdrawn. After a 4 week washout, only bromfenac sodium hydrate See end of article for authors’ affiliations ophthalmic solution was administered to the eyes of the NSAID group for 2 weeks. During the study ....................... period, ophthalmic examination, including IOP measurement was performed in an observer masked fashion. Correspondence to: Results: Kenji Kashiwagi, MD, Before initiation of bromfenac sodium hydrate, baseline IOPs of the non-NSAID group and the Department of NSAID group were 15.73 (SD 1.97) mm Hg and 15.86 (2.06) mm Hg, respectively (p=0.88). -

Update on the Mechanism of Action of Topical Prostaglandins for Intraocular Pressure Reduction Carol B
SURVEY OF OPHTHALMOLOGY VOLUME 53 SUPPLEMENT 1 NOVEMBER 2008 Update on the Mechanism of Action of Topical Prostaglandins for Intraocular Pressure Reduction Carol B. Toris, PhD,1 B’Ann T. Gabelt, MS,2 and Paul L. Kaufman, MD2 1Department of Ophthalmology and Visual Sciences, University of Nebraska Medical Center, Omaha, Nebraska, USA; and 2Department of Ophthalmology and Visual Sciences, University of Wisconsin, Madison, Wisconsin, USA Abstract. A decade has passed since the first topical prostaglandin analog was prescribed to reduce intraocular pressure (IOP) for the treatment of glaucoma. Now four prostaglandin analogs are available for clinical use around the world and more are in development. The three most efficacious of these drugs are latanoprost, travoprost, and bimatoprost, and their effects on IOP and aqueous humor dynamics are similar. A consistent finding is a substantial increase in uveoscleral outflow and a less consistent finding is an increase in trabecular outflow facility. Aqueous flow appears to be slightly stimulated as well. Prostaglandin receptors and their associated mRNAs have been located in the trabecular meshwork, ciliary muscle, and sclera, providing evidence that endogenous prostaglandins have a functional role in aqueous humor drainage. Earlier evidence found that topical PG analogs release endogenous prostaglandins. One well-studied mechanism for the enhancement of outflow by prostaglandins is the regulation of matrix metalloproteinases and remodeling of extracellular matrix. Other proposed mechanisms include widening of the connective tissue-filled spaces and changes in the shape of cells. All of these mechanisms alter the permeability of tissues of the outflow pathways leading to changes in outflow resistance and/or outflow rates.