Unoprostone Isopropyl (Rescula) Reference Number: HIM.PA.11 Effective Date: 09.04.18 Last Review Date: 11.19 Revision Log Line of Business: HIM
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Blood-Aqueous Barrier Changes After the Use of Prostaglandin Analogues in Patients with Pseudophakia and Aphakia a 6-Month Randomized Trial
CLINICAL SCIENCES Blood-Aqueous Barrier Changes After the Use of Prostaglandin Analogues in Patients With Pseudophakia and Aphakia A 6-Month Randomized Trial Enyr S. Arcieri, MD; Alessandro Santana, MD; Fabiano N. Rocha, MD; Gustavo L. Guapo, MD; Vital P. Costa, MD Objectives: To investigate the effects of prostaglandin throughout follow-up (P Ͻ .02). Four latanoprost- analogues on the blood-aqueous barrier and to evaluate treated eyes, 1 bimatoprost-treated eye, and 1 travoprost- the occurrence of cystoid macular edema in aphakic or treated eye developed cystoid macular edema; all cases pseudophakic patients with glaucoma. resolved after discontinuation of the prostaglandin ana- logue and treatment with topical diclofenac sodium. Mean Methods: In this randomized, masked-observer, 6-month intraocular pressure reductions after 6 months were higher clinical trial, patients with primary open-angle, pseudo- for the latanoprost (26%), bimatoprost (28%), and tra- phakic, or aphakic glaucoma were treated once daily with voprost (29%) groups than for the control (3%) and uno- bimatoprost (n=16), latanoprost (n=15), or travoprost prostone (14%) groups (PϽ.05). Bimatoprost induced (n=17) or twice daily with unoprostone (n=16) or lu- significantly higher hyperemia scores than latanoprost, bricant drops (control group) (n=16). Blood-aqueous bar- unoprostone, and placebo (PϽ.01). rier status, which was assessed using a laser flare meter; intraocular pressure; the occurrence of angiographic cys- toid macular edema; and conjunctival hyperemia were Conclusion: Bimatoprost, latanoprost, and travoprost use evaluated. may lead to disruption of the blood-aqueous barrier in patients with pseudophakia and aphakia. Results: Mean flare values were significantly higher in the bimatoprost, latanoprost, and travoprost groups Arch Ophthalmol. -

New York State Medicaid Drug Utilization Board Meeting Agenda
New York State Medicaid Drug Utilization Board Meeting Agenda The Drug Utilization (DUR) Board will meet April 24, 2014, from 9:00 a.m. to 4:30 p.m., Meeting Room 6, Concourse, Empire State Plaza, Albany, New York Agenda Items Preferred Drug Program (PDP) The DUR Board will review therapeutic classes, described and listed below, as they pertain to the PDP. The therapeutic classes to be reviewed contain new relevant clinical and/or financial information. Therapeutic classes not included on this agenda may be reviewed at a later date. The Board will review new clinical and financial information as required, to recommend preferred and non-preferred drugs. ^ The Board will only consider clinical information which is new since the previous review of the therapeutic class and then consider financial information. New clinical information may include a new drug or drug product information, new indications, new safety information or new published clinical trials (comparative evidence is preferred, or placebo controlled when no head-to-head trials are available). Information in abstract form alone, posters, or unpublished data are poor quality evidence for the purpose of re-review and submission is discouraged. Those wishing to submit new clinical information must do so in an electronic format by April 9, 2014 or the Board may not have ample time to review the information. ^ The current preferred and non-preferred status of drugs subject to the Preferred Drug List (PDL) may be viewed at https://newyork.fhsc.com/downloads/providers/NYRx_PDP_PDL.pdf -

Effect of Latanoprost Or 8-Iso Prostaglandin E2 Alone and in Combination on Intraocular Pressure in Glaucomatous Monkey Eyes
LABORATORY SCIENCES Effect of Latanoprost or 8-iso Prostaglandin E2 Alone and in Combination on Intraocular Pressure in Glaucomatous Monkey Eyes Rong-Fang Wang, MD; Steven M. Podos, MD; Janet B. Serle, MD; Thomas W. Mittag, PhD; F. Ventosa, MD; Bernard Becker, MD Objective: To evaluate the possible additivity of the ef- duction of IOP of 4.0 ± 0.6 mm Hg was produced when fects of latanoprost and 8-iso prostaglandin E2 (8-iso PGE2) 8-iso PGE2 was added to latanoprost and of 3.0 ± 0.7 on intraocular pressure (IOP) in monkey eyes with laser- mm Hg was produced when latanoprost was added to 8-iso induced glaucoma. PGE2 on day 13 before the morning dosing. Combina- tion therapy with both agents caused maximum IOP re- Methods: The IOP was measured hourly for 6 hours be- ductions from baseline of 11.3 ± 3.0 mm Hg (33%) (P,.05) ginning at 9:30 AM on day 1 (baseline day), days 6 and 7 (latanoprost with 8-iso PGE2 added) and of 9.8 ± 1.3 , (single-agent therapy), and days 13 and 14 (combination mm Hg (31%) (P .01) (8-iso PGE2 with latanoprost added) therapy with both agents). Following 1 day of baseline mea- on day 14. surement, 4 monkeys with unilateral glaucoma received monotherapy twice daily with either 1 drop of 0.005% la- Conclusion: Latanoprost and 8-iso PGE2 have an addi- tanoprost, or 0.1% 8-iso PGE2, 25 µL, at 9:30 AM and 3:30 tive effect on IOP in glaucomatous monkey eyes. -
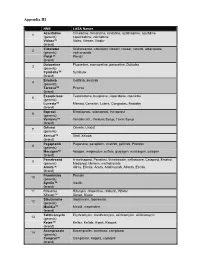
Appendix III
Appendix III NME LASA Names Azacitidine Cimetidine, famotidine, ranitidine, azathioprine, azulfidine, 1 (generic) capecitabine, zalcitabine Vidaza™ Videx, Vitrase, Viadur (brand) Cilostazol Sulfisoxazole, colestipol, clozaril, cozaar, inositol, aldactazide, 2 (generic) voriconazole Pletal™ Plendil (brand) Duloxetine Fluoxetine, atomoxetine, paroxetine, Dulcolax 3 (generic) Cymbalta™ Symbyax (brand) Erlotinib Gefitinib, imatinib 4 (generic) Tarceva™ Pexeva (brand) Eszopiclone Testolactone, buspirone, risperidone, ropinirole 5 (generic) Lunesta™ Menest, Cenestin, Lutera, Congestac, Nestabs (brand) Iloprost Bimatoprost, latanoprost, travoprost 6 (generic) Ventavis™ Ventolin inh., Ventuss Syrup, Tavist Syrup (brand) Orlistat Orlenta, Uristat 7 (generic) Xenical™ Xiral, Xeloda (brand) Pegaptanib Paganone, paraplatin, imatinib, gefitinib, Protonix 8 (generic) Macugen™ Adagen, magnesium sulfate, glucagen, mustargen, salagen (brand) Pemetrexed A-methapred, Penetrex, trimetrexate, ceftriaxone, Cetapred, Emetrol, 9 (generic) Medipred, Merrem, methotrexate Alimta™ Alinia, Elimite, Aceta, Adalimunab, Alfenta, Esclim (brand) Pramlintide Prandin 10 (generic) Symlin™ Insulin (brand) 11 Rifaximin Rifampin, rifapentine, rifabutin, Rifater Xifaxan™ Xanax, Biaxin Sibutramine Imipramine, topiramate 12 (generic) Meridia™ Mexitil, meperidine (brand) Telithromycin Erythromycin, clarithromycin, azithromycin, dirithromycin 13 (generic) Ketek™ Keflex, Keftab, K-pek, Kaopek (brand) Acamprosate Bacampicillin, acarbose, camptosar 14 (generic) Campral™ Camptosar, Keppra, -

Adverse Periocular Reactions to Five Types of Prostaglandin Analogs
Eye (2012) 26, 1465–1472 & 2012 Macmillan Publishers Limited All rights reserved 0950-222X/12 www.nature.com/eye 1 1 1 1 Adverse periocular K Inoue , M Shiokawa , R Higa , M Sugahara , CLINICAL STUDY T Soga1, M Wakakura1 and G Tomita2 reactions to five types of prostaglandin analogs Abstract Purpose We investigated the appearance explained to patients before PG frequency of eyelid pigmentation and administration. eyelash bristles after the use of five types of Eye (2012) 26, 1465–1472; doi:10.1038/eye.2012.195; prostaglandin (PG) analogs. published online 5 October 2012 Methods This study included 250 eyes from 250 patients diagnosed with primary open- Keywords: prostaglandin analogs; adverse angle glaucoma or ocular hypertension who reactions; eyelid pigmentation; eyelash bristles; were treated with either latanoprost, patient’s subjective evaluation; physician’s travoprost, tafluprost, bimatoprost, or subjective evaluation isopropyl unoprostone for 43 months in only one eye. Photographs of both eyes were obtained, and the images were assessed by Introduction three ophthalmologists who were masked to treatment type. The existence of eyelid Prostaglandin (PG) analogs are the primary pigmentation and eyelash bristles was treatment for glaucoma because of their judged, and images of the left and right eyes powerful intraocular pressure (IOP) decreasing were compared. Subjective symptoms effect, few systematic adverse reactions, and regarding the existence of eyelid convenience of once a day administration (other pigmentation and eyelash bristles were than isopropyl unoprostone (unoprostone)).1 Five types of PG analogs (latanoprost, investigated through a questionnaire. 1Inouye Eye Hospital, Tokyo, Results There was no significant difference travoprost, tafluprost, bimatoprost, and Japan between the five types of medications with unoprostone) are currently available in Japan. -

Comparison of Travoprost and Bimatoprost Plus Timolol Fixed
Comparison of Travoprost and Bimatoprost plus Timolol Fixed Combinations in Open-Angle Glaucoma Patients Previously Treated with Latanoprost plus Timolol Fixed Combination MARCO CENTOFANTI, FRANCESCO ODDONE, STEFANO GANDOLFI, ANTON HOMMER, ANDREAS BOEHM, LUCIA TANGA, CHIARA SANGERMANI, VITO SPORTELLI, MICHAEL HAUSTEIN, GIANLUCA MANNI, AND LUCA ROSSETTI ● PURPOSE: To compare the ocular hypotensive effect of ● CONCLUSIONS: Bimatoprost plus timolol and tra- bimatoprost plus timolol and travoprost plus timolol fixed voprost plus timolol can provide additional IOP-lowering combinations in glaucoma patients whose disease was effect in patients not fully controlled with latanoprost controlled but had not reached their target intraocular plus timolol. The observed additional IOP reduction was pressure (IOP) with the fixed combination of latanoprost greater with bimatoprost plus timolol with a similar plus timolol. tolerability profile. (Am J Ophthalmol 2010;150: (.DESIGN: A2؋ 3-month, multicenter, prospective, 575–580. © 2010 by Elsevier Inc. All rights reserved ● randomized, double-masked, cross-over clinical trial. ● METHODS: Eighty-nine open-angle glaucoma (OAG) OWERING INTRAOCULAR PRESSURE (IOP) IS THE patients were included. After a 6-week run-in period only evidence-based method for treating glaucoma1–3 with latanoprost plus timolol, patients were randomized and has been shown to reduce risk of visual field to either travoprost plus timolol or bimatoprost plus L progression from 13% to 19% per 1 mm Hg of IOP timolol for 3 months. Patients then switched to the lowering.2–4 According to the European Glaucoma Soci- opposite therapy for 3 additional months. The primary ety Guidelines, topical monotherapy is the first step in the end point was the comparison of mean daily IOP after 3 medical management5 and prostaglandin analogs are the months of each treatment. -

Travatan, INN-Travoprost
SCIENTIFIC DISCUSSION This module reflects the initial scientific discussion for the approval of Travatan. This scientific discussion has been updated until 1 November 2003. For information on changes after this date please refer to module 8B. 1. Introduction Travoprost is a prostaglandin analogue intended for use to reduce intraocular pressure (IOP) in patients with open-angle glaucoma or ocular hypertension. The product is presented in a concentration of 40 µg/ml of preserved eye drops (0.004%). The dose is one drop of Travoprost Eye Drops in the conjunctival sac of the affected eye(s) once daily. Travoprost Eye Drops contains travoprost (AL- 6221), a prostaglandin analogue, in a sterile ophthalmic solution formulation and it is a selective, full agonist for the FP prostanoid receptor. FP receptor agonists are reported to reduce IOP by increasing uveoscleral outflow. Rationale for the product Glaucoma is the leading cause of irreversible blindness in the world. It is a frequent disease and it has been estimated that 66.8 million have glaucoma, 6.7 million of whom are bilaterally blind. Open angle glaucoma is the most common type, mainly primary but also, in some cases, open angle glaucoma is secondary to the exfoliation syndrome or other primary ocular diseases. Glaucoma is an optic neuropathy that leads to loss of optic-nerve tissue with an excavation of the ophthalmoscopically visible optic nerve head and consequently, to a progressive loss of vision. The elevated IOP is the main risk factor for its development and reduction of IOP has been demonstrated to protect against further damage to the optic nerve, even in patients with IOP that is statistically "normal" (so called normal tension glaucoma). -

Unoprostone As Adjunctive Therapy to Timolol: a Double Masked
592 CLINICAL SCIENCE Br J Ophthalmol: first published as 10.1136/bjo.87.5.592 on 1 May 2003. Downloaded from Unoprostone as adjunctive therapy to timolol: a double masked randomised study versus brimonidine and dorzolamide A Hommer, for The Unoprostone Adjunctive Therapy Study Group,* B Kapik, N Shams ............................................................................................................................. Br J Ophthalmol 2003;87:592–598 *Members listed at the end Aims: To compare the safety and efficacy of unoprostone, brimonidine, and dorzolamide as adjunc- of paper. tive therapy to timolol in patients with primary open angle glaucoma or ocular hypertension. Methods: This was a randomised, double masked, parallel group, multicentre (14) study. After using timolol maleate 0.5% monotherapy twice a day for 2 weeks, patients (n = 146) with an early morning intraocular pressure (IOP) between 22 and 28 mm Hg, inclusively, received unoprostone isopropyl 0.15% (n = 50), brimonidine tartrate 0.2% (n = 48), or dorzolamide hydrochloride 2.0% (n = 48) twice daily as adjunctive therapy to timolol maleate 0.5% for another 12 weeks. Safety was based on comprehensive ophthalmic examinations, adverse events, and vital signs. Efficacy was based on mean change from baseline in the 8 hour diurnal IOP at week 12. Baseline was defined as values obtained See end of article for after 2 weeks of timolol monotherapy. authors’ affiliations Results: Each drug was safe and well tolerated. Burning/stinging was the most common treatment ....................... emergent adverse event. No clinically relevant changes from baseline were observed for any ophthal- Correspondence to: mic examination or vital signs. At week 12, each adjunctive therapy produced statistically significant Naveed Shams, MD, PhD, (p<0.001) reductions from timolol treated baseline in the mean 8 hour diurnal IOP (−2.7 mm Hg, uno- Novartis Ophthalmics Inc, prostone; −2.8 mm Hg, brimonidine; −3.1 mm Hg, dorzolamide). -

Additive Effect of Unoprostone and Latanoprost in Patients with Elevated Intraocular Pressure
75 CLINICAL SCIENCE Br J Ophthalmol: first published as 10.1136/bjo.86.1.75 on 1 January 2002. Downloaded from Additive effect of unoprostone and latanoprost in patients with elevated intraocular pressure Tin Aung, Paul T K Chew, Francis T S Oen, Yiong-Huak Chan, Lennard H Thean, Leonard Yip, Boon-Ang Lim, Steve K L Seah ............................................................................................................................. Br J Ophthalmol 2002;86:75–79 Aims: To assess the additive effect of unoprostone and latanoprost in patients with primary open angle glaucoma (POAG) or ocular hypertension (OHT) Methods: 32 patients with POAG or OHT were randomised to receive either latanoprost once daily or unoprostone twice daily for 4 weeks. After 4 weeks, all patients received both latanoprost and unoprostone for another 4 weeks. The IOP was measured at 9 am and 5 pm on the baseline, day 28, and day 56 visits, and at 9 am on day 14 and day 42 visits. The medications were given to the patients in an open label fashion. The observer was masked to the treatment given. The mean of the measure- ments was calculated. Safety parameters were also recorded. The additive effect of the medications was assessed by the reduction in intraocular pressure (IOP) when both medications were used, compared with when one medication was used. Results: 28 patients completed both treatment periods and had IOP data available for evaluation. See end of article for After 1 month of treatment, latanoprost significantly reduced IOP (mean by 6.1 (SEM 0.8) mm Hg authors’ affiliations (p<0.001) and unoprostone by 4.9 (1.0) mm Hg (p<0.001) from the baseline of 24.4 (0.6) mm Hg ...................... -

Bimatoprost 0.03%/Timolol 0.5% Preservative-Free Ophthalmic
BJO Online First, published on March 25, 2014 as 10.1136/bjophthalmol-2013-304064 Clinical science Br J Ophthalmol: first published as 10.1136/bjophthalmol-2013-304064 on 25 March 2014. Downloaded from Bimatoprost 0.03%/timolol 0.5% preservative-free ophthalmic solution versus bimatoprost 0.03%/timolol 0.5% ophthalmic solution (Ganfort) for glaucoma or ocular hypertension: a 12-week randomised controlled trial Ivan Goldberg,1 Rafael Gil Pina,2 Aitor Lanzagorta-Aresti,3 Rhett M Schiffman,4 Charlie Liu,4 Marina Bejanian4 ▸ Additional material is ABSTRACT As topical medications are normally dispensed published online only. To view Aim To compare the efficacy and safety of single-dose from multiuse bottles, preservative (typically ben- please visit the journal online (http://dx.doi.org/10.1136/ bimatoprost 0.03%/timolol 0.5% preservative-free (PF) zalkonium chloride, BAK) is employed to maintain bjophthalmol-2013-304064). ophthalmic solution with bimatoprost 0.03%/timolol sterility. Although many patients use preservative- 0.5% ophthalmic solution in patients with open-angle containing medications without adverse effects,10 11 1Department of Ophthalmology, University of glaucoma or ocular hypertension. some become sensitive to ophthalmic preserva- 12 13 Sydney, Sydney Eye Hospital, Methods In this multicentre, randomised, parallel- tives. A single-dose, preservative-free (PF) oph- Sydney, Australia group study, patients were randomised to bimatoprost/ thalmic fixed combination would benefit this patient 2 Clinica Oftalmologica Rafael timolol -

NDA 21-257 Page 1 TRAVATAN™™ (Travoprost Ophthalmic Solution
NDA 21-257 Page 1 TRAVATANä (travoprost ophthalmic solution) 0.004% Sterile DESCRIPTION Travoprost is a synthetic prostaglandin F2a analogue. Its chemical name is isopropyl (Z)-7- [(1R,2R,3R,5S)-3,5-dihydroxy-2-[(1E,3R)-3-hydroxy-4-[(a,a,a- trifluoro-m-tolyl)oxy]-1- butenyl]cyclopentyl]-5-heptenoate. It has a molecular formula of C26H35F3O6 and a molecular weight of 500.56. The chemical structure of travoprost is: Travoprost is a clear, colorless to slightly yellow oil that is very soluble in acetonitrile, methanol, octanol, and chloroform. It is practically insoluble in water. TRAVATANä Ophthalmic Solution 0.004% is supplied as sterile, buffered aqueous solution of travoprost with a pH of approximately 6.0 and an osmolality of approximately 290 mOsmol/kg. Each mL of TRAVATANä 0.004% contains 40 mg travoprost. Benzalkonium chloride 0.015% is added as a preservative. Inactive Ingredients are: polyoxyl 40 hydrogenated castor oil, tromethamine, boric acid, mannitol, edetate disodium, sodium hydroxide and/or hydrochloric acid (to adjust pH) and purified water. CLINICAL PHARMACOLOGY Mechanism of Action Travoprost free acid is a selective FP prostanoid receptor agonist which is believed to reduce intraocular pressure by increasing uveoscleral outflow. The exact mechanism of action is unknown at this time. Pharmacokinetics/Pharmacodynamics Absorption: Travoprost is absorbed through the cornea. In humans, peak plasma concentrations of travoprost free acid (25 pg/mL or less) were reached within 30 minutes following topical ocular administration and was rapidly eliminated. NDA 21-257 Page 2 Metabolism: Travoprost, an isopropyl ester prodrug, is hydrolyzed by esterases in the cornea to its biologically active free acid. -

Effects of Topical Antiglaucoma Medications on Corneal Epithelium As Evaluated by Gene Expression Patterns Yuka Okada, MD, Phd
JOBNAME: corn 26#Suppl. 1 2007 PAGE: 1 OUTPUT: Thursday July 26 14:59:59 2007 tsp/corn/146793/ICO200709 ARTICLE Effects of Topical Antiglaucoma Medications on Corneal Epithelium as Evaluated by Gene Expression Patterns Yuka Okada, MD, PhD Key Words: cyclooxygenase-2, antiglaucoma medication, corneal Purpose: To examine the expression pattern of the stress-related epithelium, transcription factor genes c-fos and c-jun, which encode the 2 major components of activator protein (AP)-1, and cyclooxygenase (COX)-2 in rat corneal (Cornea 2007;26(Suppl. 1):S46–S54) epithelium treated with topical antiglaucoma medications and benzalkonium chloride (BAK) preservative. Methods: Eighty-eight male Wistar rats were used. We instilled antiglaucoma eye drops (0.5% Timoptol, 0.005% Xalatan, or 0.12% opical b-blocker (timolol, 0.5% Timoptol) and prosta- Rescula), their chemical constituents (active ingredients), or BAK Tglandin (PG)F2a analogs (latanoprost, Xalatan; isopropyl preservative (0.005%, 0.01%, or 0.02%) in 1 eye of each rat. Fellow unoprostone, Rescula) are widely used in the management of eyes served as controls. The eyes were enucleated after various glaucoma and ocular hypertension. Because antiglaucoma intervals. In situ hybridization and immunohistochemistry were used drugs are used for long time periods, chronic side effects are to detect expression of c-fos, c-jun, and COX-2. a major concern. Among these side effects, ocular surface disorders attributable to the drug itself or to drug preser- Results: Expression of c-fos, c-jun, and COX-2 was minimally vatives are relatively common.1–4 Benzalkonium chloride observed in uninjured rat corneal epithelium.