Download Product Insert (PDF)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

202514Orig1s000
CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 202514Orig1s000 CLINICAL PHARMACOLOGY AND BIOPHARMACEUTICS REVIEW(S) OFFICE OF CLINICAL PHARMACOLOGY REVIEW NDA: 202-514 Submission Date(s): January 7, 2011 Proposed Brand Name TBD Generic Name Tafluprost Primary Reviewer Yongheng Zhang, Ph.D. Team Leader Philip M. Colangelo, Pharm.D., Ph.D. OCP Division DCP4 OND Division DTOP Applicant MERCK & CO., Inc. Relevant IND(s) 062690 Submission Type; Code 1S(NME) Formulation; Strength(s) Tafluprost 0.0015% Ophthalmic Solution Indication For the reduction of elevated intraocular pressure in open-angle glaucoma or ocular hypertension Dosage and Administration One drop of Tafluprost 0.0015% ophthalmic solution in the conjunctival sac of the affected eye(s) once daily in the evening TABLE OF CONTENTS 1. EXECUTIVE SUMMARY .................................................................................................................. 2 1.1. RECOMMENDATION ....................................................................................................................... 3 1.2. PHASE IV COMMITMENTS............................................................................................................. 3 1.3. SUMMARY OF IMPORTANT CLINICAL PHARMACOLOGY AND BIOPHARMACEUTICS FINDINGS.. 3 2. QUESTION BASED REVIEW ...........................................................................................................4 2.1. GENERAL ATTRIBUTES OF THE DRUG ......................................................................................... -

Blood-Aqueous Barrier Changes After the Use of Prostaglandin Analogues in Patients with Pseudophakia and Aphakia a 6-Month Randomized Trial
CLINICAL SCIENCES Blood-Aqueous Barrier Changes After the Use of Prostaglandin Analogues in Patients With Pseudophakia and Aphakia A 6-Month Randomized Trial Enyr S. Arcieri, MD; Alessandro Santana, MD; Fabiano N. Rocha, MD; Gustavo L. Guapo, MD; Vital P. Costa, MD Objectives: To investigate the effects of prostaglandin throughout follow-up (P Ͻ .02). Four latanoprost- analogues on the blood-aqueous barrier and to evaluate treated eyes, 1 bimatoprost-treated eye, and 1 travoprost- the occurrence of cystoid macular edema in aphakic or treated eye developed cystoid macular edema; all cases pseudophakic patients with glaucoma. resolved after discontinuation of the prostaglandin ana- logue and treatment with topical diclofenac sodium. Mean Methods: In this randomized, masked-observer, 6-month intraocular pressure reductions after 6 months were higher clinical trial, patients with primary open-angle, pseudo- for the latanoprost (26%), bimatoprost (28%), and tra- phakic, or aphakic glaucoma were treated once daily with voprost (29%) groups than for the control (3%) and uno- bimatoprost (n=16), latanoprost (n=15), or travoprost prostone (14%) groups (PϽ.05). Bimatoprost induced (n=17) or twice daily with unoprostone (n=16) or lu- significantly higher hyperemia scores than latanoprost, bricant drops (control group) (n=16). Blood-aqueous bar- unoprostone, and placebo (PϽ.01). rier status, which was assessed using a laser flare meter; intraocular pressure; the occurrence of angiographic cys- toid macular edema; and conjunctival hyperemia were Conclusion: Bimatoprost, latanoprost, and travoprost use evaluated. may lead to disruption of the blood-aqueous barrier in patients with pseudophakia and aphakia. Results: Mean flare values were significantly higher in the bimatoprost, latanoprost, and travoprost groups Arch Ophthalmol. -

Eyelash Growth Induced by Topical Prostaglandin Analogues, Bimatoprost, Tafluprost, Travoprost, and Latanoprost in Rabbits
JOURNAL OF OCULAR PHARMACOLOGY AND THERAPEUTICS ORIGINAL ARTICLE Volume 00, Number 0, 2013 ª Mary Ann Liebert, Inc. DOI: 10.1089/jop.2013.0075 Eyelash Growth Induced by Topical Prostaglandin Analogues, Bimatoprost, Tafluprost, Travoprost, and Latanoprost in Rabbits Ama´lia Turner Giannico,1 Leandro Lima,1 Heloisa Helena Abil Russ,2 and Fabiano Montiani-Ferreira1 Abstract Purpose: Prostaglandin analogues (PGA) are ocular hypotensive agents used for the treatment of glaucoma. Hypertrichosis of the eyelashes has been reported in humans as a side effect. Eyelash growth was investigated with clinical trials in people using bimatoprost. Scattered reports of eyelash growth during the treatment of glaucoma with other PGA are also found in the literature. We investigated the effect of 4 different topical PGA on eyelash length. Methods: Forty New Zealand white rabbits were divided into 4 groups and received daily topical application of bimatoprost, tafluprost, travoprost, and latanoprost in the left eye for 4 weeks. The right eye received no treatment. Eyelash length was measured in both eyes before and after treatment using a stainless steel digital caliper. Results: Bimatoprost and tafluprost groups had significant increases in eyelash length. We did not observe significant eyelash growth in rabbits receiving travoprost and latanoprost after 1 month of treatment. Conclusions: Today, only bimatoprost is approved for growing eyelashes, and our research shows that ta- fluprost could be further explored by the cosmetic and pharmaceutical industry. Additional research using travoprost and latanoprost as agents for eyelash growth should be performed in the future using prolonged treatment periods to determinate whether or not these PGA induce eyelash growth, and investigate other possible side effects. -

New York State Medicaid Drug Utilization Board Meeting Agenda
New York State Medicaid Drug Utilization Board Meeting Agenda The Drug Utilization (DUR) Board will meet April 24, 2014, from 9:00 a.m. to 4:30 p.m., Meeting Room 6, Concourse, Empire State Plaza, Albany, New York Agenda Items Preferred Drug Program (PDP) The DUR Board will review therapeutic classes, described and listed below, as they pertain to the PDP. The therapeutic classes to be reviewed contain new relevant clinical and/or financial information. Therapeutic classes not included on this agenda may be reviewed at a later date. The Board will review new clinical and financial information as required, to recommend preferred and non-preferred drugs. ^ The Board will only consider clinical information which is new since the previous review of the therapeutic class and then consider financial information. New clinical information may include a new drug or drug product information, new indications, new safety information or new published clinical trials (comparative evidence is preferred, or placebo controlled when no head-to-head trials are available). Information in abstract form alone, posters, or unpublished data are poor quality evidence for the purpose of re-review and submission is discouraged. Those wishing to submit new clinical information must do so in an electronic format by April 9, 2014 or the Board may not have ample time to review the information. ^ The current preferred and non-preferred status of drugs subject to the Preferred Drug List (PDL) may be viewed at https://newyork.fhsc.com/downloads/providers/NYRx_PDP_PDL.pdf -

Effect of Latanoprost Or 8-Iso Prostaglandin E2 Alone and in Combination on Intraocular Pressure in Glaucomatous Monkey Eyes
LABORATORY SCIENCES Effect of Latanoprost or 8-iso Prostaglandin E2 Alone and in Combination on Intraocular Pressure in Glaucomatous Monkey Eyes Rong-Fang Wang, MD; Steven M. Podos, MD; Janet B. Serle, MD; Thomas W. Mittag, PhD; F. Ventosa, MD; Bernard Becker, MD Objective: To evaluate the possible additivity of the ef- duction of IOP of 4.0 ± 0.6 mm Hg was produced when fects of latanoprost and 8-iso prostaglandin E2 (8-iso PGE2) 8-iso PGE2 was added to latanoprost and of 3.0 ± 0.7 on intraocular pressure (IOP) in monkey eyes with laser- mm Hg was produced when latanoprost was added to 8-iso induced glaucoma. PGE2 on day 13 before the morning dosing. Combina- tion therapy with both agents caused maximum IOP re- Methods: The IOP was measured hourly for 6 hours be- ductions from baseline of 11.3 ± 3.0 mm Hg (33%) (P,.05) ginning at 9:30 AM on day 1 (baseline day), days 6 and 7 (latanoprost with 8-iso PGE2 added) and of 9.8 ± 1.3 , (single-agent therapy), and days 13 and 14 (combination mm Hg (31%) (P .01) (8-iso PGE2 with latanoprost added) therapy with both agents). Following 1 day of baseline mea- on day 14. surement, 4 monkeys with unilateral glaucoma received monotherapy twice daily with either 1 drop of 0.005% la- Conclusion: Latanoprost and 8-iso PGE2 have an addi- tanoprost, or 0.1% 8-iso PGE2, 25 µL, at 9:30 AM and 3:30 tive effect on IOP in glaucomatous monkey eyes. -

Efficacy and Safety of the Fixed Combinations of Tafluprost/Timolol
www.nature.com/scientificreports OPEN Efcacy and safety of the fxed combinations of tafuprost/timolol and latanoprost/carteolol Received: 4 February 2019 Masahiro Fuwa, Atsushi Shimazaki, Masafumi Mieda, Naoko Yamashita, Takahiro Akaishi, Accepted: 7 May 2019 Takazumi Taniguchi & Masatomo Kato Published: xx xx xxxx In this study, we made a comparative efcacy and safety assessment of two diferent fxed combinations of drugs, viz., tafuprost/timolol (TAF/TIM) and latanoprost/carteolol (LAT/CAR), by determining their efects on intraocular pressure (IOP) in ocular normotensive monkeys and examining their toxic efects on ocular surface using human corneal epithelial cells. TAF/TIM was found to be more efective in lowering IOP for a longer duration compared to LAT/CAR. We found that the diference in the intensity of IOP-lowering efect was because of the diferences in the strength of timolol compared with that of carteolol as a beta-adrenergic antagonist and strength of tafuprost compared with that of latanoprost as a prostaglandin analogue. In addition, TAF/TIM showed much less cytotoxic efects compared to LAT/CAR on the human corneal epithelial cells. Our fndings showed that TAF/TIM is better than LAT/CAR with regard to the IOP-lowering efect in monkeys and toxicity on ocular surface. Glaucoma is a neurodegenerative disease of the eyes characterised by selective retinal ganglion cell loss, fol- lowed by progressive defects in visual feld, resulting in the principal cause of irreversible blindness worldwide1–4. Elevated intraocular pressure (IOP) is an important contributor for the progression of glaucoma, for which the current treatment primarily involves IOP reduction1,5–8. -

Review Article Analysis of the Responsiveness of Latanoprost, Travoprost, Bimatoprost, and Tafluprost in the Treatment of OAG/OHT Patients
Hindawi Journal of Ophthalmology Volume 2021, Article ID 5586719, 12 pages https://doi.org/10.1155/2021/5586719 Review Article Analysis of the Responsiveness of Latanoprost, Travoprost, Bimatoprost, and Tafluprost in the Treatment of OAG/OHT Patients Ziyan Cai ,1 Mengdan Cao,1 Ke Liu,1 and Xuanchu Duan 2 1Department of Ophthalmology, e Second Xiangya Hospital of Central South University, Changsha, Hunan, China 2Department of Ophthalmology, Changsha Aier Eye Hospital, Changsha, Hunan, China Correspondence should be addressed to Xuanchu Duan; [email protected] Received 22 February 2021; Accepted 18 May 2021; Published 25 May 2021 Academic Editor: Enrique Menc´ıa-Gutie´rrez Copyright © 2021 Ziyan Cai et al. -is is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Aim. Within the clinical setting, some patients have been identified as lacking in response to PGAs. -is meta-analysis study aimed to evaluate the responsiveness of latanoprost, travoprost, bimatoprost, and tafluprost in OAG/OHT patients, latanoprost nonresponders (LNRs), and the IOP-reducing efficacy and safety. Methods. A literature search was conducted on PubMed, Embase, and the Cochrane Controlled Trials Register. -e primary clinical endpoint was the number of responders at the end of the study. -e secondary clinical endpoint was the IOP reduction at the endpoint from baseline. Safety evaluation included five common adverse events: conjunctival hyperemia, hypertrichosis, ocular burning, ocular itching, and foreign-body sensation. Results. Eleven articles containing ten RCTs were included in this meta-analysis study. -e results highlighted that, in the OAG/ OHT population, there was no statistically significant difference in the responsiveness of the four PGAs. -

Unoprostone Isopropyl (Rescula) Reference Number: HIM.PA.11 Effective Date: 09.04.18 Last Review Date: 11.19 Revision Log Line of Business: HIM
Clinical Policy: Unoprostone Isopropyl (Rescula) Reference Number: HIM.PA.11 Effective Date: 09.04.18 Last Review Date: 11.19 Revision Log Line of Business: HIM See Important Reminder at the end of this policy for important regulatory and legal information. Description Unoprostone isopropyl ophthalmic solution, 0.15% (Rescula®) is a synthetic analog (docosanoid) of prostaglandin F2-alpha. FDA Approved Indication(s) Rescula is indicated for the reduction of elevated intraocular pressure in patients with open-angle glaucoma or ocular hypertension. Policy/Criteria Provider must submit documentation (such as office chart notes, lab results or other clinical information) supporting that member has met all approval criteria. It is the policy of health plans affiliated with Centene Corporation® that Rescula is medically necessary when the following criteria are met: I. Initial Approval Criteria A. Ocular Hypertension or Open-Angle Glaucoma (must meet all): 1. Diagnosis of open-angle glaucoma or ocular hypertension; 2. Failure of prostaglandin analogues (e.g., latanoprost, travoprost, bimatoprost) in combination with beta blockers (e.g., timolol), unless contraindicated or clinically significant adverse effects are experienced; 3. Age ≥ 18 years; 4. Dose does not exceed 1 bottle per 35 days. Approval duration: 12 months B. Other diagnoses/indications 1. Refer to the off-label use policy for the relevant line of business if diagnosis is NOT specifically listed under section III (Diagnoses/Indications for which coverage is NOT authorized): HIM.PHAR.21 for health insurance marketplace. II. Continued Therapy A. Ocular Hypertension or Open-Angle Glaucoma (must meet all): 1. Currently receiving medication via Centene benefit or member has previously met initial approval criteria; 2. -

Prostaglandin Analogs for Glaucoma
Prostaglandin Analogs Company Brand Generic Name Name Akorn Inc. Zioptan™ Tafluprost ophthalmic solution 0.0015% (PF) Allergan Inc. Durysta™ Bimatoprost 10 mcg implant Allergan Inc. Lumigan® Bimatoprost 0.01%, 0.03% Bausch & Lomb, Vyzulta™ Latanoprostene bunod 0.024% Inc. Novartis Travatan® Z Travaprost 0.004% Pfizer Inc. Xalatan® Latanoprost 0.005% Sun Ophthalmics Xelpros™ Latanoprost ophthalmic emulsion 0.005% Prostaglandin analogs work by increasing the outflow of intraocular fluid from the eye. They have few systemic side effects but are associated with changes to the eye itself, including change in iris color and growth of eyelashes. Depending on the individual, one brand of this type of medication may be more effective and produce fewer side effects. Prostaglandin analogs are taken as eye drops (except Durysta™ which is an implant). They are effective at reducing intraocular pressure in people who have open-angle glaucoma. Latanoprost and some formulations of bimatoprost and travoprost are available in generic form. Tafluprost is a preservative-free prostaglandin analog. Side Effects Side effects can include eye color change, darkening of eyelid skin, eyelash growth, droopy eyelids, sunken eyes, stinging, eye redness, and itching. Follow these steps to make it easier to put in eye drops: Preparing the Drops ● Always wash your hands before handling your eye drops or touching your eyes. ● If you’re wearing contact lenses, take them out — unless your ophthalmologist has told you to leave them in. ● Shake the drops vigorously before using them. ● Remove the cap of the eye drop medication. ○ Do not touch the dropper tip. Putting in Eye Drops ● Tilt your head back slightly and look up. -
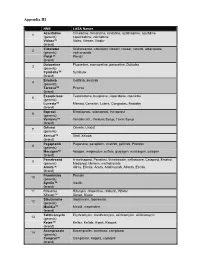
Appendix III
Appendix III NME LASA Names Azacitidine Cimetidine, famotidine, ranitidine, azathioprine, azulfidine, 1 (generic) capecitabine, zalcitabine Vidaza™ Videx, Vitrase, Viadur (brand) Cilostazol Sulfisoxazole, colestipol, clozaril, cozaar, inositol, aldactazide, 2 (generic) voriconazole Pletal™ Plendil (brand) Duloxetine Fluoxetine, atomoxetine, paroxetine, Dulcolax 3 (generic) Cymbalta™ Symbyax (brand) Erlotinib Gefitinib, imatinib 4 (generic) Tarceva™ Pexeva (brand) Eszopiclone Testolactone, buspirone, risperidone, ropinirole 5 (generic) Lunesta™ Menest, Cenestin, Lutera, Congestac, Nestabs (brand) Iloprost Bimatoprost, latanoprost, travoprost 6 (generic) Ventavis™ Ventolin inh., Ventuss Syrup, Tavist Syrup (brand) Orlistat Orlenta, Uristat 7 (generic) Xenical™ Xiral, Xeloda (brand) Pegaptanib Paganone, paraplatin, imatinib, gefitinib, Protonix 8 (generic) Macugen™ Adagen, magnesium sulfate, glucagen, mustargen, salagen (brand) Pemetrexed A-methapred, Penetrex, trimetrexate, ceftriaxone, Cetapred, Emetrol, 9 (generic) Medipred, Merrem, methotrexate Alimta™ Alinia, Elimite, Aceta, Adalimunab, Alfenta, Esclim (brand) Pramlintide Prandin 10 (generic) Symlin™ Insulin (brand) 11 Rifaximin Rifampin, rifapentine, rifabutin, Rifater Xifaxan™ Xanax, Biaxin Sibutramine Imipramine, topiramate 12 (generic) Meridia™ Mexitil, meperidine (brand) Telithromycin Erythromycin, clarithromycin, azithromycin, dirithromycin 13 (generic) Ketek™ Keflex, Keftab, K-pek, Kaopek (brand) Acamprosate Bacampicillin, acarbose, camptosar 14 (generic) Campral™ Camptosar, Keppra, -

Summary of Product Characteristics, Labelling and Package Leaflet
SUMMARY OF PRODUCT CHARACTERISTICS, LABELLING AND PACKAGE LEAFLET Taptiqom-sd-qrd-en-common-proposed-December-2020 1 SUMMARY OF PRODUCT CHARACTERISTICS Taptiqom-sd-qrd-en-common-proposed-December-2020 2 1. NAME OF THE MEDICINAL PRODUCT Taptiqom 15 micrograms/ml + 5 mg/ml eye drops, solution in single-dose container. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One ml solution contains: tafluprost 15 micrograms and timolol (as maleate) 5 mg. One single-dose container (0.3 ml) of eye drops, solution, contains 4.5 micrograms of tafluprost and 1.5 mg of timolol. One drop (about 30 µl) contains about 0.45 micrograms of tafluprost and 0.15 mg of timolol. Excipient with known effect: One ml of eye drops solution contains 1.3 mg phosphates and one drop contains approximately 0.04 mg phosphates. For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Eye drops, solution in single-dose container (eye drops). A clear, colourless solution with a pH of 6.0-6.7 and an osmolality of 290-370 mOsm/kg. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications Reduction of intraocular pressure (IOP) in adult patients with open angle glaucoma or ocular hypertension who are insufficiently responsive to topical monotherapy with beta-blockers or prostaglandin analogues and require a combination therapy, and who would benefit from preservative free eye drops. 4.2 Posology and method of administration Posology Recommended therapy is one eye drop in the conjunctival sac of the affected eye(s) once daily. If one dose is missed, treatment should continue with the next dose as planned. -

Adverse Periocular Reactions to Five Types of Prostaglandin Analogs
Eye (2012) 26, 1465–1472 & 2012 Macmillan Publishers Limited All rights reserved 0950-222X/12 www.nature.com/eye 1 1 1 1 Adverse periocular K Inoue , M Shiokawa , R Higa , M Sugahara , CLINICAL STUDY T Soga1, M Wakakura1 and G Tomita2 reactions to five types of prostaglandin analogs Abstract Purpose We investigated the appearance explained to patients before PG frequency of eyelid pigmentation and administration. eyelash bristles after the use of five types of Eye (2012) 26, 1465–1472; doi:10.1038/eye.2012.195; prostaglandin (PG) analogs. published online 5 October 2012 Methods This study included 250 eyes from 250 patients diagnosed with primary open- Keywords: prostaglandin analogs; adverse angle glaucoma or ocular hypertension who reactions; eyelid pigmentation; eyelash bristles; were treated with either latanoprost, patient’s subjective evaluation; physician’s travoprost, tafluprost, bimatoprost, or subjective evaluation isopropyl unoprostone for 43 months in only one eye. Photographs of both eyes were obtained, and the images were assessed by Introduction three ophthalmologists who were masked to treatment type. The existence of eyelid Prostaglandin (PG) analogs are the primary pigmentation and eyelash bristles was treatment for glaucoma because of their judged, and images of the left and right eyes powerful intraocular pressure (IOP) decreasing were compared. Subjective symptoms effect, few systematic adverse reactions, and regarding the existence of eyelid convenience of once a day administration (other pigmentation and eyelash bristles were than isopropyl unoprostone (unoprostone)).1 Five types of PG analogs (latanoprost, investigated through a questionnaire. 1Inouye Eye Hospital, Tokyo, Results There was no significant difference travoprost, tafluprost, bimatoprost, and Japan between the five types of medications with unoprostone) are currently available in Japan.