Additive Effect of Unoprostone and Latanoprost in Patients with Elevated Intraocular Pressure
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

202514Orig1s000
CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 202514Orig1s000 CLINICAL PHARMACOLOGY AND BIOPHARMACEUTICS REVIEW(S) OFFICE OF CLINICAL PHARMACOLOGY REVIEW NDA: 202-514 Submission Date(s): January 7, 2011 Proposed Brand Name TBD Generic Name Tafluprost Primary Reviewer Yongheng Zhang, Ph.D. Team Leader Philip M. Colangelo, Pharm.D., Ph.D. OCP Division DCP4 OND Division DTOP Applicant MERCK & CO., Inc. Relevant IND(s) 062690 Submission Type; Code 1S(NME) Formulation; Strength(s) Tafluprost 0.0015% Ophthalmic Solution Indication For the reduction of elevated intraocular pressure in open-angle glaucoma or ocular hypertension Dosage and Administration One drop of Tafluprost 0.0015% ophthalmic solution in the conjunctival sac of the affected eye(s) once daily in the evening TABLE OF CONTENTS 1. EXECUTIVE SUMMARY .................................................................................................................. 2 1.1. RECOMMENDATION ....................................................................................................................... 3 1.2. PHASE IV COMMITMENTS............................................................................................................. 3 1.3. SUMMARY OF IMPORTANT CLINICAL PHARMACOLOGY AND BIOPHARMACEUTICS FINDINGS.. 3 2. QUESTION BASED REVIEW ...........................................................................................................4 2.1. GENERAL ATTRIBUTES OF THE DRUG ......................................................................................... -

Blood-Aqueous Barrier Changes After the Use of Prostaglandin Analogues in Patients with Pseudophakia and Aphakia a 6-Month Randomized Trial
CLINICAL SCIENCES Blood-Aqueous Barrier Changes After the Use of Prostaglandin Analogues in Patients With Pseudophakia and Aphakia A 6-Month Randomized Trial Enyr S. Arcieri, MD; Alessandro Santana, MD; Fabiano N. Rocha, MD; Gustavo L. Guapo, MD; Vital P. Costa, MD Objectives: To investigate the effects of prostaglandin throughout follow-up (P Ͻ .02). Four latanoprost- analogues on the blood-aqueous barrier and to evaluate treated eyes, 1 bimatoprost-treated eye, and 1 travoprost- the occurrence of cystoid macular edema in aphakic or treated eye developed cystoid macular edema; all cases pseudophakic patients with glaucoma. resolved after discontinuation of the prostaglandin ana- logue and treatment with topical diclofenac sodium. Mean Methods: In this randomized, masked-observer, 6-month intraocular pressure reductions after 6 months were higher clinical trial, patients with primary open-angle, pseudo- for the latanoprost (26%), bimatoprost (28%), and tra- phakic, or aphakic glaucoma were treated once daily with voprost (29%) groups than for the control (3%) and uno- bimatoprost (n=16), latanoprost (n=15), or travoprost prostone (14%) groups (PϽ.05). Bimatoprost induced (n=17) or twice daily with unoprostone (n=16) or lu- significantly higher hyperemia scores than latanoprost, bricant drops (control group) (n=16). Blood-aqueous bar- unoprostone, and placebo (PϽ.01). rier status, which was assessed using a laser flare meter; intraocular pressure; the occurrence of angiographic cys- toid macular edema; and conjunctival hyperemia were Conclusion: Bimatoprost, latanoprost, and travoprost use evaluated. may lead to disruption of the blood-aqueous barrier in patients with pseudophakia and aphakia. Results: Mean flare values were significantly higher in the bimatoprost, latanoprost, and travoprost groups Arch Ophthalmol. -

Differential Activity and Clinical Utility of Latanoprost in Glaucoma and Ocular Hypertension
Clinical Ophthalmology Dovepress open access to scientific and medical research Open Access Full Text Article REVIEW Differential activity and clinical utility of latanoprost in glaucoma and ocular hypertension Fernanda Pacella Background: The purpose of this study was to demonstrate the hypotensive efficacy and tolerability Paolo Turchetti of latanoprost when used as monotherapy and as polytherapy associated with antiglaucomatous Valentina Santamaria medication proven to be ineffective in keeping intraocular pressure under control. David Impallara Methods: Three hundred and thirty-seven patients (672 eyes) affected by primary open-angle Gianpaolo Smaldone glaucoma and intraocular hypertension were recruited over a period of 10 years from the Chiara Brillante Glaucoma Centre, Department of Ophthalmological Sciences, University of Rome “Sapienza”, and treated, subject to informed consent, with latanoprost 0.005% alone or in combination Aloisa Librando with other ocular hypotensive drugs. The patients were followed during this period at regular Angela Damiano intervals, with determination of visual field, fundus oculi, visual acuity, and eventual onset of Jose Pecori-Giraldi local and systemic side effects. Elena Pacella Results: Latanoprost used as monotherapy and as polytherapy renders possible optimal and Department of Sense Organs, durable control of intraocular pressure in the form of one antiglaucomatous drug because it can University of Rome “Sapienza”, substitute for one or more drugs and obtain the same hypotensive effect. Roma, -

New York State Medicaid Drug Utilization Board Meeting Agenda
New York State Medicaid Drug Utilization Board Meeting Agenda The Drug Utilization (DUR) Board will meet April 24, 2014, from 9:00 a.m. to 4:30 p.m., Meeting Room 6, Concourse, Empire State Plaza, Albany, New York Agenda Items Preferred Drug Program (PDP) The DUR Board will review therapeutic classes, described and listed below, as they pertain to the PDP. The therapeutic classes to be reviewed contain new relevant clinical and/or financial information. Therapeutic classes not included on this agenda may be reviewed at a later date. The Board will review new clinical and financial information as required, to recommend preferred and non-preferred drugs. ^ The Board will only consider clinical information which is new since the previous review of the therapeutic class and then consider financial information. New clinical information may include a new drug or drug product information, new indications, new safety information or new published clinical trials (comparative evidence is preferred, or placebo controlled when no head-to-head trials are available). Information in abstract form alone, posters, or unpublished data are poor quality evidence for the purpose of re-review and submission is discouraged. Those wishing to submit new clinical information must do so in an electronic format by April 9, 2014 or the Board may not have ample time to review the information. ^ The current preferred and non-preferred status of drugs subject to the Preferred Drug List (PDL) may be viewed at https://newyork.fhsc.com/downloads/providers/NYRx_PDP_PDL.pdf -

Effect of Prostanoids on Human Platelet Function: an Overview
International Journal of Molecular Sciences Review Effect of Prostanoids on Human Platelet Function: An Overview Steffen Braune, Jan-Heiner Küpper and Friedrich Jung * Institute of Biotechnology, Molecular Cell Biology, Brandenburg University of Technology, 01968 Senftenberg, Germany; steff[email protected] (S.B.); [email protected] (J.-H.K.) * Correspondence: [email protected] Received: 23 October 2020; Accepted: 23 November 2020; Published: 27 November 2020 Abstract: Prostanoids are bioactive lipid mediators and take part in many physiological and pathophysiological processes in practically every organ, tissue and cell, including the vascular, renal, gastrointestinal and reproductive systems. In this review, we focus on their influence on platelets, which are key elements in thrombosis and hemostasis. The function of platelets is influenced by mediators in the blood and the vascular wall. Activated platelets aggregate and release bioactive substances, thereby activating further neighbored platelets, which finally can lead to the formation of thrombi. Prostanoids regulate the function of blood platelets by both activating or inhibiting and so are involved in hemostasis. Each prostanoid has a unique activity profile and, thus, a specific profile of action. This article reviews the effects of the following prostanoids: prostaglandin-D2 (PGD2), prostaglandin-E1, -E2 and E3 (PGE1, PGE2, PGE3), prostaglandin F2α (PGF2α), prostacyclin (PGI2) and thromboxane-A2 (TXA2) on platelet activation and aggregation via their respective receptors. Keywords: prostacyclin; thromboxane; prostaglandin; platelets 1. Introduction Hemostasis is a complex process that requires the interplay of multiple physiological pathways. Cellular and molecular mechanisms interact to stop bleedings of injured blood vessels or to seal denuded sub-endothelium with localized clot formation (Figure1). -

Effect of Latanoprost Or 8-Iso Prostaglandin E2 Alone and in Combination on Intraocular Pressure in Glaucomatous Monkey Eyes
LABORATORY SCIENCES Effect of Latanoprost or 8-iso Prostaglandin E2 Alone and in Combination on Intraocular Pressure in Glaucomatous Monkey Eyes Rong-Fang Wang, MD; Steven M. Podos, MD; Janet B. Serle, MD; Thomas W. Mittag, PhD; F. Ventosa, MD; Bernard Becker, MD Objective: To evaluate the possible additivity of the ef- duction of IOP of 4.0 ± 0.6 mm Hg was produced when fects of latanoprost and 8-iso prostaglandin E2 (8-iso PGE2) 8-iso PGE2 was added to latanoprost and of 3.0 ± 0.7 on intraocular pressure (IOP) in monkey eyes with laser- mm Hg was produced when latanoprost was added to 8-iso induced glaucoma. PGE2 on day 13 before the morning dosing. Combina- tion therapy with both agents caused maximum IOP re- Methods: The IOP was measured hourly for 6 hours be- ductions from baseline of 11.3 ± 3.0 mm Hg (33%) (P,.05) ginning at 9:30 AM on day 1 (baseline day), days 6 and 7 (latanoprost with 8-iso PGE2 added) and of 9.8 ± 1.3 , (single-agent therapy), and days 13 and 14 (combination mm Hg (31%) (P .01) (8-iso PGE2 with latanoprost added) therapy with both agents). Following 1 day of baseline mea- on day 14. surement, 4 monkeys with unilateral glaucoma received monotherapy twice daily with either 1 drop of 0.005% la- Conclusion: Latanoprost and 8-iso PGE2 have an addi- tanoprost, or 0.1% 8-iso PGE2, 25 µL, at 9:30 AM and 3:30 tive effect on IOP in glaucomatous monkey eyes. -

Efficacy and Safety of the Fixed Combinations of Tafluprost/Timolol
www.nature.com/scientificreports OPEN Efcacy and safety of the fxed combinations of tafuprost/timolol and latanoprost/carteolol Received: 4 February 2019 Masahiro Fuwa, Atsushi Shimazaki, Masafumi Mieda, Naoko Yamashita, Takahiro Akaishi, Accepted: 7 May 2019 Takazumi Taniguchi & Masatomo Kato Published: xx xx xxxx In this study, we made a comparative efcacy and safety assessment of two diferent fxed combinations of drugs, viz., tafuprost/timolol (TAF/TIM) and latanoprost/carteolol (LAT/CAR), by determining their efects on intraocular pressure (IOP) in ocular normotensive monkeys and examining their toxic efects on ocular surface using human corneal epithelial cells. TAF/TIM was found to be more efective in lowering IOP for a longer duration compared to LAT/CAR. We found that the diference in the intensity of IOP-lowering efect was because of the diferences in the strength of timolol compared with that of carteolol as a beta-adrenergic antagonist and strength of tafuprost compared with that of latanoprost as a prostaglandin analogue. In addition, TAF/TIM showed much less cytotoxic efects compared to LAT/CAR on the human corneal epithelial cells. Our fndings showed that TAF/TIM is better than LAT/CAR with regard to the IOP-lowering efect in monkeys and toxicity on ocular surface. Glaucoma is a neurodegenerative disease of the eyes characterised by selective retinal ganglion cell loss, fol- lowed by progressive defects in visual feld, resulting in the principal cause of irreversible blindness worldwide1–4. Elevated intraocular pressure (IOP) is an important contributor for the progression of glaucoma, for which the current treatment primarily involves IOP reduction1,5–8. -

Unoprostone Isopropyl (Rescula) Reference Number: HIM.PA.11 Effective Date: 09.04.18 Last Review Date: 11.19 Revision Log Line of Business: HIM
Clinical Policy: Unoprostone Isopropyl (Rescula) Reference Number: HIM.PA.11 Effective Date: 09.04.18 Last Review Date: 11.19 Revision Log Line of Business: HIM See Important Reminder at the end of this policy for important regulatory and legal information. Description Unoprostone isopropyl ophthalmic solution, 0.15% (Rescula®) is a synthetic analog (docosanoid) of prostaglandin F2-alpha. FDA Approved Indication(s) Rescula is indicated for the reduction of elevated intraocular pressure in patients with open-angle glaucoma or ocular hypertension. Policy/Criteria Provider must submit documentation (such as office chart notes, lab results or other clinical information) supporting that member has met all approval criteria. It is the policy of health plans affiliated with Centene Corporation® that Rescula is medically necessary when the following criteria are met: I. Initial Approval Criteria A. Ocular Hypertension or Open-Angle Glaucoma (must meet all): 1. Diagnosis of open-angle glaucoma or ocular hypertension; 2. Failure of prostaglandin analogues (e.g., latanoprost, travoprost, bimatoprost) in combination with beta blockers (e.g., timolol), unless contraindicated or clinically significant adverse effects are experienced; 3. Age ≥ 18 years; 4. Dose does not exceed 1 bottle per 35 days. Approval duration: 12 months B. Other diagnoses/indications 1. Refer to the off-label use policy for the relevant line of business if diagnosis is NOT specifically listed under section III (Diagnoses/Indications for which coverage is NOT authorized): HIM.PHAR.21 for health insurance marketplace. II. Continued Therapy A. Ocular Hypertension or Open-Angle Glaucoma (must meet all): 1. Currently receiving medication via Centene benefit or member has previously met initial approval criteria; 2. -

Prostaglandin Analogs for Glaucoma
Prostaglandin Analogs Company Brand Generic Name Name Akorn Inc. Zioptan™ Tafluprost ophthalmic solution 0.0015% (PF) Allergan Inc. Durysta™ Bimatoprost 10 mcg implant Allergan Inc. Lumigan® Bimatoprost 0.01%, 0.03% Bausch & Lomb, Vyzulta™ Latanoprostene bunod 0.024% Inc. Novartis Travatan® Z Travaprost 0.004% Pfizer Inc. Xalatan® Latanoprost 0.005% Sun Ophthalmics Xelpros™ Latanoprost ophthalmic emulsion 0.005% Prostaglandin analogs work by increasing the outflow of intraocular fluid from the eye. They have few systemic side effects but are associated with changes to the eye itself, including change in iris color and growth of eyelashes. Depending on the individual, one brand of this type of medication may be more effective and produce fewer side effects. Prostaglandin analogs are taken as eye drops (except Durysta™ which is an implant). They are effective at reducing intraocular pressure in people who have open-angle glaucoma. Latanoprost and some formulations of bimatoprost and travoprost are available in generic form. Tafluprost is a preservative-free prostaglandin analog. Side Effects Side effects can include eye color change, darkening of eyelid skin, eyelash growth, droopy eyelids, sunken eyes, stinging, eye redness, and itching. Follow these steps to make it easier to put in eye drops: Preparing the Drops ● Always wash your hands before handling your eye drops or touching your eyes. ● If you’re wearing contact lenses, take them out — unless your ophthalmologist has told you to leave them in. ● Shake the drops vigorously before using them. ● Remove the cap of the eye drop medication. ○ Do not touch the dropper tip. Putting in Eye Drops ● Tilt your head back slightly and look up. -
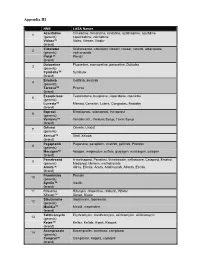
Appendix III
Appendix III NME LASA Names Azacitidine Cimetidine, famotidine, ranitidine, azathioprine, azulfidine, 1 (generic) capecitabine, zalcitabine Vidaza™ Videx, Vitrase, Viadur (brand) Cilostazol Sulfisoxazole, colestipol, clozaril, cozaar, inositol, aldactazide, 2 (generic) voriconazole Pletal™ Plendil (brand) Duloxetine Fluoxetine, atomoxetine, paroxetine, Dulcolax 3 (generic) Cymbalta™ Symbyax (brand) Erlotinib Gefitinib, imatinib 4 (generic) Tarceva™ Pexeva (brand) Eszopiclone Testolactone, buspirone, risperidone, ropinirole 5 (generic) Lunesta™ Menest, Cenestin, Lutera, Congestac, Nestabs (brand) Iloprost Bimatoprost, latanoprost, travoprost 6 (generic) Ventavis™ Ventolin inh., Ventuss Syrup, Tavist Syrup (brand) Orlistat Orlenta, Uristat 7 (generic) Xenical™ Xiral, Xeloda (brand) Pegaptanib Paganone, paraplatin, imatinib, gefitinib, Protonix 8 (generic) Macugen™ Adagen, magnesium sulfate, glucagen, mustargen, salagen (brand) Pemetrexed A-methapred, Penetrex, trimetrexate, ceftriaxone, Cetapred, Emetrol, 9 (generic) Medipred, Merrem, methotrexate Alimta™ Alinia, Elimite, Aceta, Adalimunab, Alfenta, Esclim (brand) Pramlintide Prandin 10 (generic) Symlin™ Insulin (brand) 11 Rifaximin Rifampin, rifapentine, rifabutin, Rifater Xifaxan™ Xanax, Biaxin Sibutramine Imipramine, topiramate 12 (generic) Meridia™ Mexitil, meperidine (brand) Telithromycin Erythromycin, clarithromycin, azithromycin, dirithromycin 13 (generic) Ketek™ Keflex, Keftab, K-pek, Kaopek (brand) Acamprosate Bacampicillin, acarbose, camptosar 14 (generic) Campral™ Camptosar, Keppra, -

(12) United States Patent (10) Patent No.: US 9,155.716 B2 Chang Et Al
US009 155716B2 (12) United States Patent (10) Patent No.: US 9,155.716 B2 Chang et al. (45) Date of Patent: *Oct. 13, 2015 (54) ENHANCED BIMATOPROST OPHTHALMIC (56) References Cited SOLUTION U.S. PATENT DOCUMENTS (71) Applicant: ALLERGAN, INC. Irvine, CA (US) 4,055,602 A 10, 1977 Nelson 4,100,192 A 7, 1978 Morozowich (72) Inventors: Chin-Ming Chang, Tustin, CA (US); 4,122.282 A 10, 1978 Nelson James N. Chang, Newport Beach, CA 4,123,441 A 10, 1978 Johnson 4,128,577 A 12/1978 Nelson (US); Rhett M. Schiffman, Laguna 4,171,331 A 10, 1979 Biddlecom Beach, CA (US); R. Scott Jordan, 4,183,870 A 1/1980 Caton Trabuco Canyon, CA (US); Joan-En 4,303,796 A 12/1981 Nelson Chang-Lin, Tustin, CA (US) 4,382,953. A 5, 1983 Ishii 4,543,353 A 9, 1985 Faustini 4,599,353 A 7, 1986 Bito (73) Assignee: Allergan, Inc., Irvine, CA (US) 4,812.457 A 3/1989 Narumiya 4,994,274 A 2f1991 Chan (*) Notice: Subject to any disclaimer, the term of this 5,034413 A 7, 1991 Chan patent is extended or adjusted under 35 5,281.591 A 1/1994 Burke 5,352,708 A 10, 1994 Woodward U.S.C. 154(b) by 0 days. 5,474,979 A 12/1995 Ding 5,510,383 A 4/1996 Bishop This patent is Subject to a terminal dis 5,545,665 A 8, 1996 Burk claimer. 5,587,391 A 12, 1996 Burk 5,607,978 A 3, 1997 Woodward (21) Appl. -

Adverse Periocular Reactions to Five Types of Prostaglandin Analogs
Eye (2012) 26, 1465–1472 & 2012 Macmillan Publishers Limited All rights reserved 0950-222X/12 www.nature.com/eye 1 1 1 1 Adverse periocular K Inoue , M Shiokawa , R Higa , M Sugahara , CLINICAL STUDY T Soga1, M Wakakura1 and G Tomita2 reactions to five types of prostaglandin analogs Abstract Purpose We investigated the appearance explained to patients before PG frequency of eyelid pigmentation and administration. eyelash bristles after the use of five types of Eye (2012) 26, 1465–1472; doi:10.1038/eye.2012.195; prostaglandin (PG) analogs. published online 5 October 2012 Methods This study included 250 eyes from 250 patients diagnosed with primary open- Keywords: prostaglandin analogs; adverse angle glaucoma or ocular hypertension who reactions; eyelid pigmentation; eyelash bristles; were treated with either latanoprost, patient’s subjective evaluation; physician’s travoprost, tafluprost, bimatoprost, or subjective evaluation isopropyl unoprostone for 43 months in only one eye. Photographs of both eyes were obtained, and the images were assessed by Introduction three ophthalmologists who were masked to treatment type. The existence of eyelid Prostaglandin (PG) analogs are the primary pigmentation and eyelash bristles was treatment for glaucoma because of their judged, and images of the left and right eyes powerful intraocular pressure (IOP) decreasing were compared. Subjective symptoms effect, few systematic adverse reactions, and regarding the existence of eyelid convenience of once a day administration (other pigmentation and eyelash bristles were than isopropyl unoprostone (unoprostone)).1 Five types of PG analogs (latanoprost, investigated through a questionnaire. 1Inouye Eye Hospital, Tokyo, Results There was no significant difference travoprost, tafluprost, bimatoprost, and Japan between the five types of medications with unoprostone) are currently available in Japan.