The Molecular and Morphological Systematics of Subfamily Epidendroideae (Orchidaceae)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Genus Brassavola, (L.) R.Br
The Genus Brassavola, (L.) R.Br. in W.T.Aiton, Hortus Kew. 5: 216 (1813) Type: Brassavola [B.] cucullata [bra-SAH-vo-la kyoo-kyoo-LAH-ta] There are 28 species (OrchidWiz [update Dec 2017]) that are epiphytes and sometimes lithophytes at elevations of from sea level to 3300 ft (1000 m) from Mexico, southern Caribbean islands to northern Argentina in moist or wet montane forests, mangroves, rocky crevices and cliff faces. They are most fragrant at night and many with a citrus smell. The genus is characterized by very small pencil-like pseudobulbs, often forming large clumps; a single, fleshy, apical, sub-terete leaf and the inflorescence produced form the apex of the pseudobulb. The inflorescence carries from a single to a few large flowers. The floral characteristics are elongate narrow similar sepals and petals, the base of the lip usually tightly rolled around at least a portion of the column which carries 12, sometimes eight unequal pollina with prominent opaque caudicles. The flowers usually occur, as a rule, in spring, summer and fall. The flowers are generally yellow to greenish white with a mostly white lip. It is not unusual for dark spots, usually purple, to be in the region where the sepals, petals, and lip join the stem (claw). This spotting is a dominant generic trait in Brassavola nodose. They are easily cultivated under intermediate conditions. Although this is a relatively small genus (28 species), the species show an unusually close relationship with one another in their floral patterns, coloration, and column structure making identification difficult, key to know where the plants were collected. -

Evolution of Anatomical Characters in Acianthera Section Pleurobotryae (Orchidaceae: Pleurothallidinae)
RESEARCH ARTICLE Evolution of anatomical characters in Acianthera section Pleurobotryae (Orchidaceae: Pleurothallidinae) Audia Brito Rodrigues de AlmeidaID*, Eric de Camargo SmidtID, Erika Amano Programa de PoÂs-GraduacËão em BotaÃnica, Setor de Ciências BioloÂgicas, Universidade Federal do ParanaÂ, Curitiba, PR, Brazil * [email protected] a1111111111 a1111111111 a1111111111 a1111111111 Abstract a1111111111 Acianthera section Pleurobotryae is one of ten sections of the genus Acianthera and include four species endemic to the Atlantic Forest. The objective of this study was to describe com- paratively the anatomy of vegetative organs and floral micromorphology of all species of Acianthera section Pleurobotryae in order to identify diagnostic characters between them OPEN ACCESS and synapomorphies for the section in relation of other sections of the genus. We analyzed Citation: Almeida ABRd, Smidt EdC, Amano E roots, ramicauls, leaves and flowers of 15 species, covering eight of the nine sections of (2019) Evolution of anatomical characters in Acianthera section Pleurobotryae (Orchidaceae: Acianthera, using light microscopy and scanning electron microscopy. Acianthera section Pleurothallidinae). PLoS ONE 14(3): e0212677. Pleurobotryae is a monophyletic group and the cladistic analyses of anatomical and flower https://doi.org/10.1371/journal.pone.0212677 micromorphology data, combined with molecular data, support internal relationship hypoth- Editor: Suzannah Rutherford, Fred Hutchinson eses among the representatives of this section. The synapomorphies identified for A. sect. Cancer Research Center, UNITED STATES Pleurobotryae are based on leaf anatomy: unifacial leaves, round or elliptical in cross-sec- Received: August 24, 2018 tion, round leaves with vascular bundles organized in concentric circles, and mesophyll with Accepted: February 7, 2019 28 to 30 cell layers. -

Actes Du 15E Colloque Sur Les Orchidées De La Société Française D’Orchidophilie
Cah. Soc. Fr. Orch., n° 7 (2010) – Actes 15e colloque de la Société Française d’Orchidophilie, Montpellier Actes du 15e colloque sur les Orchidées de la Société Française d’Orchidophilie du 30 mai au 1er juin 2009 Montpellier, Le Corum Comité d’organisation : Daniel Prat, Francis Dabonneville, Philippe Feldmann, Michel Nicole, Aline Raynal-Roques, Marc-Andre Selosse, Bertrand Schatz Coordinateurs des Actes Daniel Prat & Bertrand Schatz Affiche du Colloque : Conception : Francis Dabonneville Photographies de Francis Dabonneville & Bertrand Schatz Cahiers de la Société Française d’Orchidophilie, N° 7, Actes du 15e Colloque sur les orchidées de la Société Française d’Orchidophilie. ISSN 0750-0386 © SFO, Paris, 2010 Certificat d’inscription à la commission paritaire N° 55828 ISBN 978-2-905734-17-4 Actes du 15e colloque sur les Orchidées de la Société Française d’Orchidophilie, D. Prat et B. Schatz, Coordinateurs, SFO, Paris, 2010, 236 p. Société Française d’Orchidophilie 17 Quai de la Seine, 75019 Paris Cah. Soc. Fr. Orch., n° 7 (2010) – Actes 15e colloque de la Société Française d’Orchidophilie, Montpellier Préface Ce 15e colloque marque le 40e anniversaire de notre société, celle-ci ayant vu le jour en 1969. Notre dernier colloque se tenait il y a 10 ans à Paris en 1999, 10 ans c’est long, 10 ans c’est très loin. Il fallait que la SFO renoue avec cette traditionnelle organisation de colloques, manifestation qui a contribué à lui accorder la place prépondérante qu’elle occupe au sein des orchidophiles français et de la communauté scientifique. C’est chose faite aujourd’hui. Nombreux sont les thèmes qui font l’objet de communications par des intervenants dont les compétences dans le domaine de l’orchidologie ne sont plus à prouver. -

INVENTAIRE DES ORCHIDEES DE TALATAKELY PARC NATIONAL DE RANOMAFANA ETUDES MORPHOLOGIQUE ET MOLECULAIRE DE CINQ ESPECES DU GENRE Aerangis (Rchb.F.)
UNIVERSITE D’ANTANANARIVO FACULTE DES SCIENCES Département de Biologie et Ecologie Végétales Mémoire pour l’obtention du Diplôme d’Etudes Approfondies (D.E.A.) En Biologie et Ecologie Végétales OPTION : ECOLOGIE VEGETALE INVENTAIRE DES ORCHIDEES DE TALATAKELY PARC NATIONAL DE RANOMAFANA ETUDES MORPHOLOGIQUE ET MOLECULAIRE DE CINQ ESPECES DU GENRE Aerangis (Rchb.f.) Présenté par RANDRIANINDRINA Veloarivony Rence Aimée (Maître ès Sciences) Soutenu publiquement le, 31 Janvier 2008 Devant la Commission de jury composée de : Président : Pr. RAJERIARISON Charlotte Examinateurs : Dr. RABAKONANDRIANINA Elisabeth Dr. FALINIAINA Lucien Rapporteurs : Dr. RAKOUTH Bakolimalala Dr. EDWARD Louis Jr. 1 UNIVERSITE D’ANTANANARIVO FACULTE DES SCIENCES Département de Biologie et Ecologie Végétales Mémoire pour l’obtention du Diplôme d’Etudes Approfondies (D.E.A.) En Biologie et Ecologie Végétales OPTION : ECOLOGIE VEGETALE INVENTAIRE DES ORCHIDEES DE TALATAKELY PARC NATIONAL DE RANOMAFANA ETUDES MORPHOLOGIQUE ET MOLECULAIRE DE CINQ ESPECES DU GENRE Aerangis (Rchb.f.) Présenté par RANDRIANINDRINA Veloarivony Rence Aimée (Maître ès Sciences) Soutenu publiquement le, 31 Janvier 2008 Devant la Commission de jury composée de : Président : Pr. Charlotte RAJERIARISON Examinateurs : Dr. Elisabeth RABAKONANDRIANINA Dr Lucien. FALINIAINA Rapporteurs : Dr. Bakolimalala RAKOUTH Dr. Louis Jr. EDWARD 2 REMERCIEMENTS En premier lieu, nous voudrions rendre gloire à Dieu pour sa bienveillance et sa bénédiction. Mené à terme ce mémoire, est le fruit de la collaboration entre -

Plastome Structure and Adaptive Evolution of Calanthe S.L. Species
Plastome structure and adaptive evolution of Calanthe s.l. species Yanqiong Chen, Hui Zhong, Yating Zhu, Yuanzhen Huang, Shasha Wu, Zhongjian Liu, Siren Lan and Junwen Zhai Key Laboratory of National Forestry and Grassland Administration for Orchid Conservation and Utilization at College of Landscape Architecture, Fujian Agriculture and Forestry University, Fuzhou, Fujian, China Fujian Ornamental Plant Germplasm Resources Innovation & Engineering Application Research Center, Fujian Agriculture and Forestry University, Fuzhou, Fujian, China ABSTRACT Calanthe s.l. is the most diverse group in the tribe Collabieae (Orchidaceae), which are pantropical in distribution. Illumina sequencing followed by de novo assembly was used in this study, and the plastid genetic information of Calanthe s.l. was used to investigate the adaptive evolution of this taxon. Herein, the complete plastome of five Calanthe s.l. species (Calanthe davidii, Styloglossum lyroglossa, Preptanthe rubens, Cephalantheropsis obcordata, and Phaius tankervilliae) were determined, and the two other published plastome sequences of Calanthe s.l. were added for comparative analyses to examine the evolutionary pattern of the plastome in the alliance. The seven plastomes ranged from 150,181 bp (C. delavayi) to 159,014 bp (C. davidii) in length and were all mapped as circular structures. Except for the three ndh genes (ndhC, ndhF, and ndhK ) lost in C. delavayi, the remaining six species contain identical gene orders and numbers (115 gene). Nucleotide diversity was detected across the plastomes, and we screened 14 mutational hotspot regions, including 12 non-coding regions and two gene regions. For the adaptive evolution investigation, three species showed positive selected genes compared with others, C. -

Systematics and Evolution of the Genus Pleurothallis R. Br
Systematics and evolution of the genus Pleurothallis R. Br. (Orchidaceae) in the Greater Antilles DISSERTATION zur Erlangung des akademischen Grades doctor rerum naturalium (Dr. rer. nat.) im Fach Biologie eingereicht an der Mathematisch-Naturwissenschaftlichen Fakultät I der Humboldt-Universität zu Berlin von Diplom-Biologe Hagen Stenzel geb. 05.10.1967 in Berlin Präsident der Humboldt-Universität zu Berlin Prof. Dr. J. Mlynek Dekan der Mathematisch-Naturwissenschaftlichen Fakultät I Prof. Dr. M. Linscheid Gutachter/in: 1. Prof. Dr. E. Köhler 2. HD Dr. H. Dietrich 3. Prof. Dr. J. Ackerman Tag der mündlichen Prüfung: 06.02.2004 Pleurothallis obliquipetala Acuña & Schweinf. Für Jakob und Julius, die nichts unversucht ließen, um das Zustandekommen dieser Arbeit zu verhindern. Zusammenfassung Die antillanische Flora ist eine der artenreichsten der Erde. Trotz jahrhundertelanger floristischer Forschung zeigen jüngere Studien, daß der Archipel noch immer weiße Flecken beherbergt. Das trifft besonders auf die Familie der Orchideen zu, deren letzte Bearbeitung für Cuba z.B. mehr als ein halbes Jahrhundert zurückliegt. Die vorliegende Arbeit basiert auf der lang ausstehenden Revision der Orchideengattung Pleurothallis R. Br. für die Flora de Cuba. Mittels weiterer morphologischer, palynologischer, molekulargenetischer, phytogeographischer und ökologischer Untersuchungen auch eines Florenteils der anderen Großen Antillen wird die Genese der antillanischen Pleurothallis-Flora rekonstruiert. Der Archipel umfaßt mehr als 70 Arten dieser Gattung, wobei die Zahlen auf den einzelnen Inseln sehr verschieden sind: Cuba besitzt 39, Jamaica 23, Hispaniola 40 und Puerto Rico 11 Spezies. Das Zentrum der Diversität liegt im montanen Dreieck Ost-Cuba – Jamaica – Hispaniola, einer Region, die 95 % der antillanischen Arten beherbergt, wovon 75% endemisch auf einer der Inseln sind. -

Partial Endoreplication Stimulates Diversification in the Species-Richest Lineage Of
bioRxiv preprint doi: https://doi.org/10.1101/2020.05.12.091074; this version posted May 14, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Partial endoreplication stimulates diversification in the species-richest lineage of 2 orchids 1,2,6 1,3,6 1,4,5,6 1,6 3 Zuzana Chumová , Eliška Záveská , Jan Ponert , Philipp-André Schmidt , Pavel *,1,6 4 Trávníček 5 6 1Czech Academy of Sciences, Institute of Botany, Zámek 1, Průhonice CZ-25243, Czech Republic 7 2Department of Botany, Faculty of Science, Charles University, Benátská 2, Prague CZ-12801, Czech Republic 8 3Department of Botany, University of Innsbruck, Sternwartestraße 15, 6020 Innsbruck, Austria 9 4Prague Botanical Garden, Trojská 800/196, Prague CZ-17100, Czech Republic 10 5Department of Experimental Plant Biology, Faculty of Science, Charles University, Viničná 5, Prague CZ- 11 12844, Czech Republic 12 13 6equal contributions 14 *corresponding author: [email protected] 1 bioRxiv preprint doi: https://doi.org/10.1101/2020.05.12.091074; this version posted May 14, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 15 Abstract 16 Some of the most burning questions in biology in recent years concern differential 17 diversification along the tree of life and its causes. -

Universidade Federal Do Amapá Pró-Reitoria De Graduação Campus Mazagão Curso De Licenciatura Em Educação Do Campo: Ciências Agrárias E Biologia
UNIVERSIDADE FEDERAL DO AMAPÁ PRÓ-REITORIA DE GRADUAÇÃO CAMPUS MAZAGÃO CURSO DE LICENCIATURA EM EDUCAÇÃO DO CAMPO: CIÊNCIAS AGRÁRIAS E BIOLOGIA ROSIANE DE SOUZA PIMENTEL O GÊNERO Lepanthes Sw. (ASPARAGALES: ORCHIDACEAE) PARA O BRASIL Mazagão – AP 2019 ROSIANE DE SOUZA PIMENTEL O GÊNERO Lepanthes Sw. (ASPARAGALES: ORCHIDACEAE) PARA O BRASIL Monografia de conclusão de curso apresentada ao Curso de Licenciatura em Educação do Campo: Ciências Agrárias e Biologia, da Universidade Federal do Amapá, Campus Mazagão, como requisito parcial para obtenção do grau de Licenciado. Orientador: Prof. Dr. Raullyan Borja Lima e Silva Coorientador: Prof. Dr. Patrick de Castro Cantuária Mazagão – AP 2019 Dados Internacionais de Catalogação na Publicação (CIP) Biblioteca Central da Universidade Federal do Amapá Elaborada por Orinete Costa Souza – CRB-11/920 Pimentel, Rosiane de Souza. O gênero Lepanthes Sw. (Asparagales: orchidaceae) para o Brasil / Rosiane Souza Pimentel ; Orientador, Raullyan Borja Lima e Silva ; Coorientador, Patrick de Castro Cantuária. – Mazagão, 2019. 64 f. : il. Trabalho de Conclusão de Curso (Graduação) – Fundação Universidade Federal do Amapá – Campus Mazagão, Coordenação do Curso de Educação no Campo com ênfase em Agronomia e Biologia. 1. Plantas - Análise. 2. Fanerógams. 3. Diversidades das plantas. 4. Herbários - Amapá. I. Silva, Raullyan Borja Lima e, orientador. II. Cantuária, Patrick de Castro, coorientador. III. Fundação Universidade Federal do Amapá – Campus Mazagão. V. Título. 582.13 P644g CDD: 22. ed A toda minha família, em especial, aos meus filhos. Dedico AGRADECIMENTOS Agradeço a Deus pelo dom da vida. Á Universidade Federal do Amapá e aos professores do Campus Mazagão pela oportunidade e conhecimento compartilhado. Ao prof. Dr. -
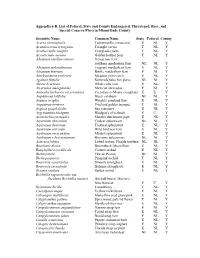
Conservation Appendix 6-B Listed Flora
Appendix 6-B. List of Federal, State and County Endangered, Threatened, Rare, and Special Concern Flora in Miami-Dade County Scientific Name Common Name State Federal County Acacia choriophylla Tamarindillo; cinnecord E NL Y Acanthocereus tetragenus Triangle cactus T NL Y Acoelorraphe wrightii Everglades palm T NL Y Acrostichum aureum Golden leather fern T NL Y Adiantum capillus-veneris Venus hair fern; southern maidenhair fern NL NL Y Adiantum melanoleucum Fragrant maidenhair fern E NL Y Adiantum tenerum Brittle maidenhair fern E NL Y Aeschynomene pratensis Meadow joint-vetch E NL Y Agalinis filifolia Seminole false fox glove NL NL Y Aletris bracteata White colic root E NL Y Alvaradoa amorphoides Mexican alvaradoa E NL Y Amorpha herbacea var.crenulata Crenulate (=Miami) leadplant E E Y Amphitecna latifolia Black calabash NL NL Y Anemia wrightii Wright's pineland fern E NL Y Angadenia berteroi Pineland golden trumpet T NL Y Argusia gnaphalodes Sea rosemary E NL Y Argythamnia blodgettii Blodgett's silverbush E C Y Aristolochia pentandra Marsh's dutchmans pipe E NL Y Asplenium abscissum Cutleaf spleenwort NL NL Y Asplenium dentatum Toothed spleenwort E NL Y Asplenium serratum Wild bird nest fern E NL Y Asplenium verecundum Modest spleenwort E NL Y Asplenium x biscaynianum Biscayne spleenwort NL NL Y Asteraea lobata Lobed croton; Florida treefern NL NL Y Baccharis dioica Broombush falsewillow E NL Y Basiphyllaea corallicola Carter's orchid E NL Y Bletia patula Flor de Pesmo NL NL Y Bletia purpurea Pinepink orchid T NL Y Bourreria cassinifolia Smooth strongback E NL Y Bourreria succulenta Bahama strongback E NL Y Brassia caudata Spider orchid E NL Y Brickellia eupatorioides var. -

August 2010 Volume 51: Number 8
The Atlanta Orchid Society Bulletin The Atlanta Orchid Society is affiliated with the American Orchid Society, the Orchid Digest Corporation and the Mid-America Orchid Congress. Newsletter Editor: Mark Reinke August 2010 www.AtlantaOrchidSociety.org Volume 51: Number 8 AUGUST MONTHLY MEETING Topic: Integrated Orchid Conservation at the Atlanta Botanical Garden Speaker: Matt Richards 8:00 pm Monday, August 9 at the Atlanta Botanical Garden, Day Hall Matt Richards graduated from The Ohio State University with a B.S. in Horticulture. Special attention was given to the study of Orchidaceae and the asymbiotic culture of orchids during his undergraduate studies. In 2006 he was hired as Orchid Center Horticulturist at the Atlanta Botanical Garden. He began working on the propagation of Georgia’s native orchids in the Ron Determann Tissue Culture lab at ABG, and has since assumed the full operating responsibilities of the tissue culture laboratory. He now holds the title Cattleya bicolor ssp. bicolor of Orchid Conservation Specialist. He has advanced the culture of native North American This bi-foliate species native to Southeast Brazil orchids, and has successfully grown plants of typically blooms in August and September in the many rare species from seed to flower. In 2007 he Northern Hemisphere. was invited to join the IUCN (World Conservation Union) as a member of the Orchid Specialist Group (North American Region) under the SSC In This Issue…… (Species Survival Commission). Page Matt’s talk will cover the ABG’s involvement in 2 AtlOS Volunteer -

Redalyc.EFFORTS to CONSERVE ENDANGERED TERRESTRIAL
Lankesteriana International Journal on Orchidology ISSN: 1409-3871 [email protected] Universidad de Costa Rica Costa Rica RANGEL-VILLAFRANCO, MONICA; ORTEGA-LARROCEA, M. PILAR EFFORTS TO CONSERVE ENDANGERED TERRESTRIAL ORCHIDS IN SITU AND EX SITU AT TWO NATURAL RESERVES WITHIN CENTRAL MEXICO Lankesteriana International Journal on Orchidology, vol. 7, núm. 1-2, marzo, 2007, pp. 326-333 Universidad de Costa Rica Cartago, Costa Rica Available in: http://www.redalyc.org/articulo.oa?id=44339813068 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative LANKESTERIANA 7(1-2): 326-333. 2007. EFFORTS TO CONSERVE ENDANGERED TERRESTRIAL ORCHIDS IN SITU AND EX SITU AT TWO NATURAL RESERVES WITHIN CENTRAL MEXICO 1 1,2 MONICA RANGEL-VILLAFRANCO & M. PILAR ORTEGA-LARROCEA 1 Departamento de Edafología, Instituto de Geología, Universidad Nacional Autónoma de México. Circuito Exterior de Ciudad Universitaria, México Distrito Federal, 04510. México. 2 Author for correspondence: [email protected] KEY WORDS: in situ conservation, ex situ conservation, orchid fungi isolation, seed banks The natural vegetation in and around Mexico City macrobulon, Epidendrum anisatu, Habenaria strictis- once harbored an unusually high number of plant and sima, Liparis greenwoodiana) (Hágsater et al. 2005). animal (insect) species, including endemics (Vázquez In contrast, in the Chichinautzin Area, eight types 1973, Ceballos & Galindo 1984, Rzedowski 1991). of vegetation can be found. An altitudinal gradient The high diversity in this region has been attributed joint with successive periods of volcanic activity to the unusual topography resulting from a series of are combined and pedogenetic processes through volcanic eruptions that ended ca. -

CITES Orchid Checklist Volumes 1, 2 & 3 Combined
CITES Orchid Checklist Online Version Volumes 1, 2 & 3 Combined (three volumes merged together as pdf files) Available at http://www.rbgkew.org.uk/data/cites.html Important: Please read the Introduction before reading this Part Introduction - OrchidIntro.pdf Part I : All names in current use - OrchidPartI.pdf (this file) Part II: Accepted names in current use - OrchidPartII.pdf Part III: Country Checklist - OrchidPartIII.pdf For the genera: Aerangis, Angraecum, Ascocentrum, Bletilla, Brassavola, Calanthe, Catasetum, Cattleya, Constantia, Cymbidium, Cypripedium, Dendrobium (selected sections only), Disa, Dracula, Encyclia, Laelia, Miltonia, Miltonioides, Miltoniopsis, Paphiopedilum, Paraphalaenopsis, Phalaenopsis, Phragmipedium, Pleione, Renanthera, Renantherella, Rhynchostylis, Rossioglossum, Sophronitella, Sophronitis Vanda and Vandopsis Compiled by: Jacqueline A Roberts, Lee R Allman, Sharon Anuku, Clive R Beale, Johanna C Benseler, Joanne Burdon, Richard W Butter, Kevin R Crook, Paul Mathew, H Noel McGough, Andrew Newman & Daniela C Zappi Assisted by a selected international panel of orchid experts Royal Botanic Gardens, Kew Copyright 2002 The Trustees of The Royal Botanic Gardens Kew CITES Secretariat Printed volumes: Volume 1 first published in 1995 - Volume 1: ISBN 0 947643 87 7 Volume 2 first published in 1997 - Volume 2: ISBN 1 900347 34 2 Volume 3 first published in 2001 - Volume 3: ISBN 1 84246 033 1 General editor of series: Jacqueline A Roberts 2 Part I: ORCHIDACEAE BINOMIALS IN CURRENT USAGE Ordered alphabetically on All