Address to the National Press Club
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

VOLUME XXIL NO. 8. RED BANK, N. J., WEDNESDAY, AUGUST 16,1899. PAGES 1 to 8. A-FAIR and FESTIVAL DEATHS Jdukingthewfiek THINGS W
VOLUME XXIL NO. 8. RED BANK, N. J., WEDNESDAY, AUGUST 16,1899. PAGES 1 TO 8. THEIR SILVER WEDDING. >8he married William Foster thirty years A-FAIR AND FESTIVAL DEATHS JDUKINGTHEWfiEK ago. She leaves five children. They THINGS WON AT A FAIR. Mr. and Mrs. Herman Koch Mar- are John-Foster, Mrs. Kate Wilmot, Mrs. A HARVEST HOME AT LITTLE ried Twenty-Five Wears. WILLIAM H. GUERNSEY DIES AT FAIR HAVEN DAUGHTERS OF Thomas F. Ga$ill and Lena and Cornelia ° ' SILVER LAST NIGHT. Last Thursday night was the twenty- LIBERTY MAKE 8100, Foster. fifth anniversary of the marriage of Mr Held hu the Women of the Metho ne was Owe of the Most Pronounced - Albert nankins. Miss Anna B. lUinton Won a Bicy- dist Church-Over Eight Hundred and Mrs, Herman Koch of Shrewsbury . ProhibittoMstu ofJUonmouth, and cle, Wm. Bennett Won a Barrel of. Persons Present and About $ftOO avenue. A number of their neighbors •ForTe'tiifr-iBe Kept Up the-Apita- Albert, son of EHPS Hankins of Mata- Potatoes and Charles Dennis Won - Cleared. ; .. and friends arranged a surprise,visit in UanatOelfora.. •.;..:;.•..' •.'[ wan, died of consumption on Monday of a Writtna Desk-Other Winnings. " ©vereighfc hundred persons attended celebration of the event. The.evening William H. Guernsey...of Ce'nterville, last week, aged 22 years. Hejiad been The fair .held -by the Fair Haven the harvest home at, Little.Silver yester- was spent with dancing, singing and in Raritan township,.died last Saturday confined to the house six weeks. He Daughters of Liberty in Monmouth hall 1 day afternoon and evening/The music. -

SI Allocations
Free TV Australia DTTB SI Register Transport Stream Service Information for Television Market Area All values are hexadecimal Issue 15 Date: October 2020 Western Australia Tasmania Northern Territory Remote Remote Queensland, Mandurah (Turner NSW, Vic, SA, Tas Perth Bunbury Albany Remote Hobart Launceston Darwin Alice Springs Northern Territory Hill) (See Note 3) (See Notes 1 and 2) (See notes 1 and 2) LCN Broadcaster Service Name SID SID SID SID SID SID SID SID SID SID SID NID NID NID NID NID NID NID NID NID NID NID TSID TSID TSID TSID TSID TSID TSID TSID TSID TSID TSID ONID ONID ONID ONID ONID ONID ONID ONID ONID ONID (dec) ONID 3201 3239 0261 1010 3256 0263 1010 3256 0263 1010 3256 0263 1010 3256 0263 1010 325B 0271 1010 3257 0273 1010 325C 0281 1010 325B 0283 ABC1 2 02E1 02E1 02E1 02E1 02E1 0271 0291 0281 02F1 ABC News 24 24 02E0 02E0 02E0 02E0 02E0 0270 0290 0280 02F0 ABC ABC1 21 02E3 02E3 02E3 02E3 02E3 0273 0293 0283 02F3 ABC2 / ABC4 22 02E2 02E2 02E2 02E2 02E2 0272 0292 0282 02F2 ABC3 23 02E4 02E4 02E4 02E4 02E4 0274 0294 0284 02F4 ABC Dig Music 200 02E6 02E6 02E6 02E6 02E6 0276 0296 0286 02F6 ABC Jazz 201 02E7 02E7 02E7 02E7 02E7 0277 0297 0287 02F7 3202 3202 0320 3202 3202 03A0 3202 3202 03A0 3202 3202 03A0 3202 3202 03A0 3202 3202 0380 3202 3202 0380 3202 3202 0360 SBS ONE 3 0321 03A1 03A1 03A1 03A1 0381 0381 0361 SBS ONE HD 30 0325 03A5 03A5 03A5 03A5 0385 0385 0365 SBS VICELAND HD 31 0326 03A6 03A6 03A6 03A6 0386 0386 0366 SBS World Movies 32 0327 03A7 03A7 03A7 03A7 0387 0387 0367 SBS Food 33 0323 03A3 03A3 03A3 03A3 0383 -

Nine Network Australia Selects Intelsat to Distribute Domestic Television Programming
Nine Network Australia Selects Intelsat to Distribute Domestic Television Programming October 24, 2016 Intelsat will provide satellite connectivity and global fiber services via the IntelsatOne network to bring news content from around the world to Australia LUXEMBOURG--(BUSINESS WIRE)--Oct. 24, 2016-- Intelsat (NYSE: I), operator of the world’s first Globalized Network, powered by its leading satellite backbone, today announced a contract with Nine Network Australia to distribute programming to the company’s six domestic television stations as well as provide global satellite and fiber connectivity using the IntelsatOne terrestrial network to bring news content and special events from around the world to Australia for its network affiliates. Nine Network Australia (NNA) is one of the country’s top-rated commercial television networks, with local stations in Sydney, Melbourne, Brisbane, Adelaide, Perth and Darwin, along with a regional broadcaster, NBN. The network also has news bureaus in Canberra, London and Los Angeles. NNA is a subsidiary of Nine Entertainment Co., one of Australia’s leading media companies. Intelsat initially is providing services via the Australia/New Zealand Ku-Band beam on Intelsat 19 for the network’s broadcasting requirement. Under the agreement, Intelsat’s network support will extend well into the next decade. “Our Globalized Network allows us to meet NNA’s needs today and to increase our level of support if the network expands over time to meet the demands of multi-platform coverage,” said Terry Bleakley, Regional Vice President, Asia Pacific Sales, Intelsat. “NNA will also benefit from Intelsat’s global coverage for international contribution and distribution requirements.” NNA previously used Intelsat for its domestic and international occasional use requirements. -

A Nine Network Gift Annuity Can Offer Big Financial Benefits for You... and a Significant Contribution to Our Region
One-Life Example: What one person can do. A Nine Network Gift Annuity Our founders were visionaries. Mrs. Edwards is 85 years old and is interested in helping the Nine Network We all want to make a positive difference in the can offer big financial benefits They believed education was essential to a healthy community and hoped continue its quality educational and entertainment programs. At the same time lives of others. But sometimes we wonder what television could bring opportunities for learning into every home. she would like to have a guaranteed lifetime income. Mrs. Edwards contributes one person can do. for you... and a significant $10,000 to the Nine Network’s Gift Annuity Program and is entitled to: contribution to our region. Remember, television was a relatively new device in 1952, when our founders were Doris E. Wolff was a graduate of St. Louis University with a master’s degree in developing the idea that would become national educational television. $780 per year, or a 7.80% return, for life. advanced math. She became a math teacher at Cleveland High School and A Federal charitable deduction of $5,600. instilled a love of math in many, many of her students over the years. Her students We believe they were successful because they were guided by their principles and $640 of each year’s income is tax-free for described her as compassionate and encouraging. She was also a Sunday School their purpose. For them, television was just a tool—the technology that would approximately 5.5 years. teacher who cared very deeply about the young people she taught. -

Portland Daily Pr Ss
PORTLAND DAILY PR SS. VOLUME II I THURSDAY PORTLAND, ME., MORNING, SEPTEMBER 24, 1863. WHOLE NO. 380. PORTLAND DAILY PRESS, est point is at 197th street, where it is a few FOR SALE & TO LET. inches below tide water. The new LEGAL & OFFICIAL. BUSINESS CARDS. JOHN T. OILMAN, Editor. reservoir EDUCATIONAL. covers 100 acre*; is about 30 feet holds BUSINESS CARDS. Is at No. EXCHANGE deep, Counting Itooin to Let. published 82* STREET, 1,000,000,000 gallons of water, and cost U. S. USarslinrs $1,500,- ROOM over No. 90 Commercial St. Sale. FRENCH LANGUAGE. IN FOX 000. Around its rim is A CARD. BLOCK, by a walk tor pedestrians; COUNTINGThomas Block, to let. Apply to United Stater ok America, l MILLINERY. N. A. FOSTER A CO. outside of that is a bridle-path, and beyond N. J. MILLER. District of Maine, ss. j a DR. that is a beautiful Five mcli71 dtf Over92 Commercial Street. to Writ of Vend: Expo: to me di- S. C. again carriage-drive. rected from tlie PROFJR HENRI DUCOM FERNALD, miles of have been PURSUANT Hod. Aflhur Ware, .Judge of 'Forms : bridle-path completed; eight the United States District ('ourt, within and for the Has miles of To Lei On and after Monday, Sept. 14th, Kesumed IiIm I ,essnns. The Portland Daily Prk«b i* published every j carriage-road; 18 miles of foot-path. District of Maine, I shall expose ana sell at Public ttEIXTIST, at $0.00 per year in It is a live-mile drive from the or low- commodious Chamber in the northerly cor. -

SENATE Official Committee Hansard
COMMONWEALTH OF AUSTRALIA SENATE Official Committee Hansard INFORMATION TECHNOLOGIES COMMITTEE Reference: Self-regulation in the information and communication industries WEDNESDAY, 22 APRIL 1998 SYDNEY BY AUTHORITY OF THE SENATE CANBERRA 1997 INTERNET The Proof and Official Hansard transcripts of Senate committee hearings, some House of Representatives committee hearings and some joint committee hearings are available on the Internet. Some House of Representatives committees and some joint committees make available only Official Hansard transcripts. The Internet address is: http://www.aph.gov.au/hansard SENATE Wednesday, 22 April 1998 SELECT COMMITTEE ON INFORMATION TECHNOLOGIES Members: Senator Ferris (Chair), Senator Quirke (Deputy Chair), Senators Calvert, Harradine, McGauran, Tierney, Reynolds and Stott Despoja Senators attending the hearing: Senator Ferris (Chair), Senator Quirke (Deputy Chair), Senators Calvert and Harradine Matter referred by the Senate for inquiry into and report on: Evaluate the appropriateness, effectiveness and privacy implications of the existing self-regulatory framework in relation to the information and communications industries and, in particular, the adequacy of the complaints regime. WITNESSES BLOCK, Ms Jessica, Corporate Counsel, Nine Network, 24 Artarmon Road, Willoughby, New South Wales 2068 ................................. 332 BRANIGAN, Mr Anthony Michael, General Manager, Federation of Australian Commercial Television Stations, 44 Avenue Road, Mosman, New South Wales 2088 ....................................................... -

PBS in St. Louis
PBS in St. Louis MEDIA 2020 KIT With a multiplatform approach, Nine Network creates meaningful connections for businesses and nonprofit organizations with our viewers and members. We reach more than 750,000 monthly viewers who want to explore the world and become engaged in civic life. SOURCE: NIELSEN IMPRESSIONS, AGE 2+, 2109. Nine Network contributes to a strong, vibrant, thriving St. Louis region by creating opportunities for citizens to learn, connect and grow. nineNetwork of Public Media Local Programming Special Reports Nine’s specials celebrate the St. Louis region’s strengths and accomplishments, uplifting those in need, and finding solutions to the challenges. Upcoming projects available on request. Living St. Louis A sample of past programs include: Since launching in 2004, the regional Emmy-winning Living St. • Now Hiring: A Skilled Workforce Louis has captured the people, places, and organizations that make our region consequential and complex. • Of Black and Blue: The Journey of African American Police • A Baseball Legacy: Fans Remember the St. Louis Browns • St. Louis Teen Talent Competition Event Sponsorship We encourage our community to explore, learn, and participate through the more than 100 events we hold every year. Nine events offer unique opportunities to connect your brand Donnybrook with broad audiences on-air, online, One of the longest-running public affairs programs on and in print, as well as reaching television, Donnybrook is not another dry, tame talk show— desirable, targeted audiences during the issues are hot and so is the discussion. Donnybrook is also stimulating events. Custom packages available on podcast. are available to fit your company’s needs and marketing goals. -

Annual Report to Shareholders
create distribute engage ifc The Year in Brief 2 Chairman’s Address 4 Chief Executive Officer’s Address 6 Divisional Results 8 Operational Review 16 Nine Cares create 17 Governance 18 Board of Directors 20 Directors’ Report distribute 25 Remuneration Report 44 Operating and Financial Review 48 Financial Report engage 108 Shareholder Information ibc Corporate Directory During FY17, Nine achieved its goal of turning the Network performance around, after a disappointing year in FY16. Momentum in Free To Air TV turned positive for Nine in Q2, and this improvement continued throughout the remainder of the financial year. The success of Nine’s broadcast content has, in turn, driven take-up and use of 9Now which has grown exponentially to over 4 million registered users, and is becoming a valuable contributor to the P&L. Nine’s Subscription Video on Demand platform Stan, has matured significantly over the past 12 months and now holds a clear number 2 position in the market. Nine’s digital publishing business has been successfully repositioned post the Microsoft relationship, laying the foundations for growth into the future. All of Nine’s businesses are built around the key content verticals of news, sport, lifestyle and entertainment. Result In Brief In FY17, on a revenue decline of 4%, Nine reported Group EBITDA of $206 million, up 2% on FY16. Driving this growth was an underlying cost decrease of 1%, and a reported cost decrease of 4% which included the Government regulated licence fee relief of $33 million. Net Profit after Tax increased by 3% to $123.6 million compared to the Pro Forma FY16 result. -
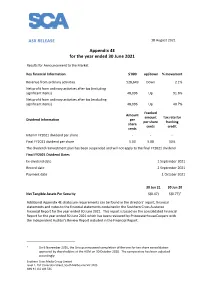
ASX RELEASE Appendix 4E for the Year Ended 30 June 2021
ASX RELEASE 18 August 2021 Appendix 4E for the year ended 30 June 2021 Results for Announcement to the Market Key Financial Information $'000 up/down % movement Revenue from ordinary activities 528,649 Down 2.1% Net profit from ordinary activities after tax (including significant items) 48,096 Up 91.6% Net profit from ordinary activities after tax (excluding significant items) 48,096 Up 40.7% Franked Amount amount Tax rate for Dividend Information per per share franking share cents credit cents Interim FY2021 dividend per share - - - Final FY2021 dividend per share 5.00 5.00 30% The dividend reinvestment plan has been suspended and will not apply to the final FY2021 dividend. Final FY2021 Dividend Dates Ex-dividend date 1 September 2021 Record date 2 September 2021 Payment date 1 October 2021 30 Jun 21 30 Jun 20 Net Tangible Assets Per Security $(0.47) $(0.77)1 Additional Appendix 4E disclosure requirements can be found in the directors’ report, financial statements and notes to the financial statements contained in the Southern Cross Austereo Financial Report for the year ended 30 June 2021. This report is based on the consolidated Financial Report for the year ended 30 June 2021 which has been reviewed by PricewaterhouseCoopers with the Independent Auditor's Review Report included in the Financial Report. 1 On 6 November 2020, the Group announced completion of the one for ten share consolidation approved by shareholders at the AGM on 30 October 2020. The comparative has been adjusted accordingly. Southern Cross Media Group Limited Level 2, 257 Clarendon Street, South Melbourne VIC 3205 ABN 91 116 024 536 Southern Cross Media Group Limited ASX release Approved for release by the Board of directors. -

UC Berkeley UC Berkeley Electronic Theses and Dissertations
UC Berkeley UC Berkeley Electronic Theses and Dissertations Title The Federalist Society and the "Structural Constitution:" An Epistemic Community At Work Permalink https://escholarship.org/uc/item/54z517w5 Author Hollis-Brusky, Amanda Publication Date 2010 Peer reviewed|Thesis/dissertation eScholarship.org Powered by the California Digital Library University of California The Federalist Society and the “Structural Constitution:” An Epistemic Community At Work by Amanda Lee Hollis-Brusky A dissertation submitted in partial satisfaction of the requirements for the degree of Doctor of Philosophy in Political Science in the Graduate Division of the University of California, Berkeley Committee in charge: Professor Robert A. Kagan, Co-Chair Professor Shannon C. Stimson, Co-Chair Professor Gordon Silverstein Professor Daniel A. Farber Spring 2010 The Federalist Society and the “Structural Constitution:” An Epistemic Community At Work © 2010 by Amanda Lee Hollis-Brusky Abstract The Federalist Society and the “Structural Constitution:” An Epistemic Community At Work by Amanda Lee Hollis-Brusky Doctor of Philosophy in Political Science University of California, Berkeley Professor Robert A. Kagan, Co-Chair Professor Shannon C. Stimson, Co-Chair This thesis contributes to an understanding of the pathways of civic influence into the least dangerous branch of American politics – the Judicial Branch. Specifically, this thesis examines the influence of the Federalist Society for Law and Public Policy – a conservative and libertarian legal network of more than 40,000 members – on twelve of the most salient Supreme Court decisions concerning federalism and the separation of powers over the last three decades. As a special case, it also examines Federalist Society influence on a subset of controversial Executive Branch policies issued under George W. -

Mediaportal Report
WED 04 SEPTEMBER 2013 Mediaportal Report Executive Summary The Sydney Town Hall clocktower media call yesterday generated 33 stories in the past 24 hours. The stories featured on all major metro newspapers, television networks and radio stations, and reached approximately 2.4 million people and were worth and estimated $359,000 in equivalent advertising spend, according to Media Monitors. TOWN HALL Wait for ring finally over MX (Sydney), Sydney, General News 03 Sep 2013 Page 6 - 63 words - ASR AUD 297 Photo: No - Type: News Item - Size: 27.47 cm² - NSW - Australia - ID: 211412058 THE Sydney Town Hall bells were sounded today for the first time in 532 days, following extensive repairs and restoration. The clocktower belfry, some 55m above George St, has been restored by experts during the past 17 months. The 129-year-old clock and its bell are operated by a car-sized mechanism and a spokeswoman said the repair was the first stage of restoring Town Hall. Copyright Agency Ltd (CAL) licensed copy View print article 97,970 CIRCULATION COPYRIGHT This report and its contents are for the internal research use of Mediaportal subscribers only and may not be provided to any third party by any means for any purpose without the express permission of iSentia and/or the relevant copyright owner. For more information contact [email protected] DISCLAIMER iSentia uses multiple audience data sources for press, internet, TV and radio, including AGB Nielsen Media Research, Audit Bureau of Circulations, comScore, CSM Media Research, OzTAM, Nielsen, Research International and TNS. For general information purposes only. -

Audience Insights Tracker As at 27 April, 2020
Audience Insights Tracker As at 27 April, 2020 Linear TV BVOD Digital & Publishing Radio Nine maintains its dominance as Dwell times on all Nine’s digital news sites 9Now continues to be the leading Audiences continue to turn to Nine Australia’s preferred demographic delivered double digital growth in CFTA BVOD platform with a year-to- Radio for trusted voices and platforms network for primetime, driven by March, a reflection of audiences turning date share of 50% in total minutes. where they can voice their own trusted news and current affairs, and to trusted news sites for up-to-the minute opinions. The second GfK Radio Survey premium entertainment content. information. Furthermore 9Now has ranked as the of 2020 has seen 2GB, 3AW, 4BC and leading CFTA BVOD platform for 15 out 6PR post significant audience growth Nine continues to reach new As audiences look to inspiration at of 17 weeks (YTD) for both live and during the COVID-19 crisis. audiences across all key demos, with home 9Honey, Traveller and Good Food VOD minutes weekly share. +1.7 reach points for P25-54 in the last are connecting with Nine Radio has had 1.45M podcast month compared to previous. Australians, providing content to indulge As viewers continue to turn to downloads to date, and a 16% their passions across health, food and entertainment for an escape during increase in downloads month to date. Extended time spent at home is travel. seeing increased engagement on the current global pandemic, family favourite LEGO Masters S2 continues to Gfk’s COVID-19 radio user survey has linear TV year-on-year, with P25-54 Nine’s Consumer Pulse survey spanning captivate audiences taking the top found that 72% of respondents trust spending 11 more minutes a week readers of the Herald, The Age, The two spots for live viewing last week.